
���� ���������л��ܼ������ñ������Ȼ�̼����ȡ���룬
��1��KIO3�������ữ��KI��Һ����������ԭ��Ӧ���ɵⵥ�ʣ�
��2��˫��ˮ����ǿ�����ԣ����������£�˫��ˮ�������������ɵⵥ�ʣ�
��3��ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲������ɫ�����������˵�����е��ʵ⣩��
��� �⣺���������л��ܼ������ñ������Ȼ�̼����ȡ���룬
�ʴ�Ϊ���������Ȼ�̼��
��1��KIO3�������ữ��KI��Һ����������ԭ��Ӧ���ɵⵥ�ʣ����ӷ�ӦΪ5I-+IO3-+6H+=3I2+3H2O���ʴ�Ϊ��5I-+IO3-+6H+=3I2+3H2O��
��2��˫��ˮ����ǿ�����ԣ����������£�˫��ˮ�������������ɵⵥ�ʣ���������ԭ����ˮ����Ӧ����ʽΪ��H2O2+2I-+2H+=I2+2H2O��
�ʴ�Ϊ��H2O2+2I-+2H+=I2+2H2O��
��3���ⵥ���������۱���ɫ���ⵥ�ʵļ��鷽����ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲������ɫ�����������˵�����е��ʵ⣩��
�ʴ�Ϊ��ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲������ɫ�����������˵�����е��ʵ⣩��
���� ���⿼�������ʵķ�����ᴿ��������ԭ��Ӧ��֪ʶ�㣬Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬�ѶȲ�����ȷ��ȡ����ѡȡ����֪����Һ������������Щ���dz�����㣮


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
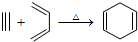 �����Ҫ�ϳ�
�����Ҫ�ϳ� ���õ�ԭʼԭ�Ͽ����ǣ�������
���õ�ԭʼԭ�Ͽ����ǣ�������| A�� | 2-��-1��3-����ϩ��2-��Ȳ | B�� | 1��3-���ϩ��2-��Ȳ | ||
| C�� | 2��3-����-1��3-���ϩ����Ȳ | D�� | 2��3-����-1��3-����ϩ��1-��Ȳ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
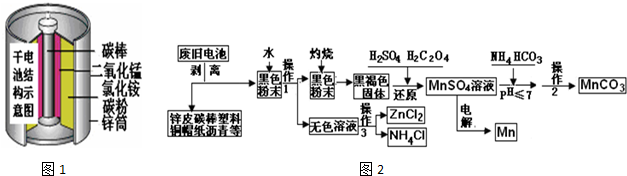
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
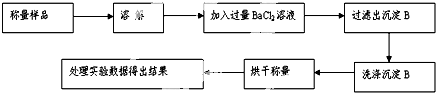
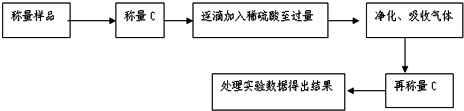

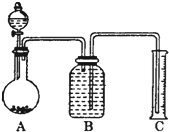
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
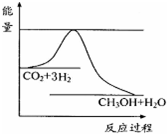 ������ʵ��д�����з�Ӧ���Ȼ�ѧ����ʽ
������ʵ��д�����з�Ӧ���Ȼ�ѧ����ʽ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �� | D�� | �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com