
| A�� | ij������Һ��pH=a��������Һϡ��10������Һ��pHֵΪb����b=a+1 | |
| B�� | CH3OOH��CH3COONa�����Һ�����ܴ��ڣ�c ��CH3COOH����c ��CH3COO-����c ��H+����c ��Na+����c ��0H-�� | |
| C�� | �����£���pH=3��H2SO4��pH=11��һԪ��BOH-��Һ�������ϣ�������Һ����Ϊ���Ի����� | |
| D�� | �����£�Ũ�Ⱦ�ΪO��1mol•L-1�Ģ�CH3COOH��Һ��NaOH��Һ��CH3OONa ��Һ�У�ˮ�ĵ���̶Ȣۣ��٣��� |
���� A������Ϊ���ᣬ��ˮϡ�ʹٽ����룻
B���纬�����������ƣ�������㣻
C����Ϊǿ���Һ�����ԣ�ΪΪ�����Ӧ��ʼ��ԣ�
D������ˮ��ٽ�ˮ�ĵ��룬�ᡢ������ˮ�ĵ��룬c ��H+����c ��0H-��Խ��ˮ�ĵ���̶�ԽС��
��� �⣺A������Ϊ���ᣬ��ˮϡ�ʹٽ����룬������Һ��pH=a��������Һϡ��10������Һ��pHֵΪb��ӦΪb��a+1����A����
B���纬�����������ƣ���Һ�����ԣ�������c ��CH3COOH����c ��CH3COO-����c ��H+����c ��Na+����c ��0H-������B����
C����Ϊǿ���Һ�����ԣ�ΪΪ�����Ӧ��ʼ��ԣ�����Ϊ���ԣ���C����
D������ˮ��ٽ�ˮ�ĵ��룬�ᡢ������ˮ�ĵ��룬c ��H+����c ��0H-��Խ��ˮ�ĵ���̶�ԽС�������Ϊ���ᣬ��ˮ�ĵ���̶Ȣۣ��٣��ڣ���D��ȷ��
��ѡD��
���� ���⿼���Ϊ�ۺϣ��漰������ʵĵ��룬���Ļ�ϼ����֪ʶ��������ѧ���ķ����ͼ��������Ŀ��飬Ϊ��Ƶ���㣬ע�����������ʵĵ����ص��Ӱ�����أ��ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A | �Ҵ���Ӧʱ�ϼ���λ | B | ���ŵ��Ӱ�� |
 ��1����Ũ���Ṳ�ȵ�170��ʱ�ڢݶϼ� ��2����Ũ���Ṳ�ȵ�140��ʱֻ�ϼ��� | ��1�����Ʒ�Ӧʱ�Ҵ���ˮ����˵�����һ�Ӱ�죬���ǻ���Hԭ�ӻ����Լ��� ��2��������Һ�м�Na0H��Һ����壬˵���ܱ���Ӱ�죬���ǻ���Hԭ�ӻ�������ǿ | ||
| C | �����ᴿ | D | ��������; |
| ��1����ҵ�Ҵ�ͨ���������ˮ�Ҵ� ��2�����л��б��ӣ�����ˮ��������ˣ���ȥ���屽�ӳ��� | ��1�����ͼ�ˮ�������� ��2����������ж�����������ɱ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���·����ޡ��顢��˿�ijɷֶ�����ά�� | |
| B�� | �ع��͵���Ҫ�ɷ��Ǹ�֬������������������Ʒ��� | |
| C�� | ʯ�ͷ����Ŀ����Ϊ�˻����ϩ����ϩ��1��3-����ϡ | |
| D�� | �Ҵ�����������ͳ��������������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
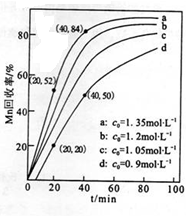
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
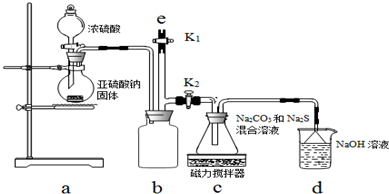
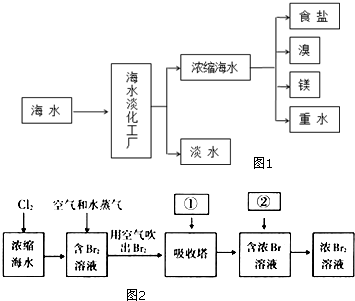 ��嫵ĺ�����һ�������Դ���⣬�̲��ŷ��ĵĿ�����DZ���Ļ�ѧ��Դ����ͼ1�Ǻ�ˮ�ӹ���ʾ��ͼ��������ͼ�ش����⣮
��嫵ĺ�����һ�������Դ���⣬�̲��ŷ��ĵĿ�����DZ���Ļ�ѧ��Դ����ͼ1�Ǻ�ˮ�ӹ���ʾ��ͼ��������ͼ�ش����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4+��S2-�� CO32- | B�� | AlO21��SO42-����MnO4- | ||
| C�� | NO3-��Cl-��SO42- | D�� | MnO4-�� SO42-����NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
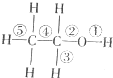
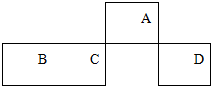 ������Ԫ��A��B��C��D��Ԫ�����ڱ��е����λ����ͼ��ʾ������A����ۺ�����۵Ĵ�������2�������жϲ���ȷ���ǣ�������
������Ԫ��A��B��C��D��Ԫ�����ڱ��е����λ����ͼ��ʾ������A����ۺ�����۵Ĵ�������2�������жϲ���ȷ���ǣ�������| A�� | CԪ���ڵؿ��еĺ�����������Ԫ�� | |
| B�� | ����������Ӧˮ��������ԣ�C��D | |
| C�� | Ԫ��A��Ԫ��B�γɵĻ�����BA��һ���������ǽ������� | |
| D�� | ʵ���ҿ���B�ĵ�����AԪ������������Ӧ��ˮ���ﷴӦ��ȡ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com