
25 ЁцЃЌ101 k PaЪБЃЌЧПЫсгыЧПМюЕФЯЁШмвКЗЂЩњжаКЭЗДгІЩњГЩ1molЫЎЪБЫљЗХГіЕФШШСПдМЮЊ57.3 kJ/molЃЛаСЭщЕФШМЩеШШЮЊ5518 kJ/molЁЃЯТСаШШЛЏбЇЗНГЬЪНЪщаДе§ШЗЕФЪЧЃЈ ЃЉ
AЃЎCH3COOH(aq)+NaOH(aq)===
CH3COONa(aq)+H2O(l)  H=
H=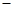 57.3kJ/mol
57.3kJ/mol
 BЃЎKOH(aq)+
BЃЎKOH(aq)+ H2SO4(aq)=
H2SO4(aq)=  K2SO4(aq)+H2O(l)
K2SO4(aq)+H2O(l)
 H=
H=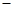 57.3kJ/mol
57.3kJ/mol
CЃЎ2C8H18(g)+25O2
(g)=16CO2 (g)+18H2O(1)  H=
H=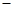 11036
kJ/mol
11036
kJ/mol
 DЃЎC8H18(l)+
DЃЎC8H18(l)+ 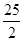 O2 (g)=8CO2 (g)+
9H2O(g)
O2 (g)=8CO2 (g)+
9H2O(g)  H=
H=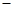 5518 kJ/mol
5518 kJ/mol

| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2012НьКгФЯЪЁНЙзїЪаИпШ§ЕквЛДЮжЪСПМьВтРэзлЛЏбЇВПЗжЃЈДјНтЮіЃЉ ЬтаЭЃКЬюПеЬт
(15Зж)жабЇЛЏбЇГЃМћВПЗждЊЫидзгНсЙЙМАаджЪШчЯТБэЫљЪОЃК
| ађКХ | дЊЫи | НсЙЙМАаджЪ |
| Ђй | A | AЕЅжЪЪЧЩњЛюжаГЃМћН№ЪєЃЌЫќгаСНжжТШЛЏЮяЃЌЯрЖдЗжзгжЪСПЯрВю35.5 |
| Ђк | B | BдзгKЁЂLЁЂMВуЕчзгЪ§жЎБШЪЧ1:4:1 |
| Ђл | C | CЪЧЛюЦУЗЧН№ЪєдЊЫиЃЌЦфЕЅжЪГЃЮТЯТГЪЦјЬЌЕЋЛЏбЇаджЪЮШЖЈ |
| Ђм | D | DЕЅжЪБЛгўЮЊЁАаХЯЂИяУќЕФДпЛЏМСЁБЃЌЪЧГЃгУЕФАыЕМЬхВФСЯ[РДдД:Z*xx*k.Com] |
| Ђн | E | ЭЈГЃЧщПіЯТЃЌEУЛгае§ЛЏКЯМлЃЌAЁЂCЁЂFЖМФмгыEаЮГЩЖўжжЛђЖўжжвдЩЯЛЏКЯЮя |
| Ђо | F | FдкжмЦкБэжаПЩвдХХдкЂёAзхЃЌвВгаШЫЬсГіХХдкЂїAзх |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014НьеуНЪЁИпЖўЩЯбЇЦкЦкФЉПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
25 ЁцЃЌ101 k PaЪБЃЌЧПЫсгыЧПМюЕФЯЁШмвКЗЂЩњжаКЭЗДгІЕФжаКЭШШЮЊ-57.3 kJ/molЃЌаСЭщЕФШМЩеШШЮЊ-5518 kJ/molЁЃЯТСаШШЛЏбЇЗНГЬЪНЪщаДе§ШЗЕФЪЧЃЈ ЃЉ
AЃЎ2H+(aq)
+ (aq)+
(aq)+ (aq)+2
(aq)+2 (aq) = BaSO4(s)+2H
(aq) = BaSO4(s)+2H O(l);
O(l);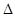 H =
H = 57.3 kJ/mol
57.3 kJ/mol
BЃЎKOH(aq)+ H
H SO4(aq) =
SO4(aq) =  K
K SO4(aq)+H
SO4(aq)+H O(l);
O(l);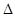 H=
H= 57.3kJ/mol
57.3kJ/mol
CЃЎC8H18(l)+
 O
O (g)=8CO
(g)=8CO (g)+ 9H
(g)+ 9H O(g);
O(g);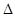 H=
H= 5518 kJ/mol
5518 kJ/mol
DЃЎ2C8H18(g)+25O (g)=16CO
(g)=16CO (g)+18H
(g)+18H O(1);
O(1);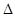 H=
H= 5518 kJ/mol
5518 kJ/mol
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013НьАВЛеЪЁИпШ§ЕквЛДЮЭГПМЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
25 ЁцЃЌ101 k PaЪБЃЌЧПЫсгыЧПМюЕФЯЁШмвКЗЂЩњжаКЭЗДгІЕФжаКЭШШЮЊ57.3 kJ/molЃЌаСЭщЕФШМЩеШШЮЊ5518 kJ/molЁЃЯТСаШШЛЏбЇЗНГЬЪНЪщаДе§ШЗЕФЪЧ ( )
AЃЎ2H+(aq)+SO42Ѓ(aq)+Ba2ЃЋ(aq)+2OH-(aq)=BaSO4(s)+2H2O(1)
ЁїH= 57.3 kJ/mol
57.3 kJ/mol
BЃЎKOH(aq)+ H2 SO4(aq)=
H2 SO4(aq)= K2SO4(aq)+H2O(l) ЁїH=
K2SO4(aq)+H2O(l) ЁїH= 57.3kJ/mol
57.3kJ/mol
CЃЎC8H18(I)+ O2(g)=8CO2(g)+9H2O ЁїH=
O2(g)=8CO2(g)+9H2O ЁїH= 5518 kJ/mol
5518 kJ/mol
DЃЎ2C8H18(g)+25O2(g)=16CO2(g)+18H2O(1)
ЁїH= 5518 kJ/mol
5518 kJ/mol
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013НьЙуЖЋЪЁИпЖўЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэ ЬтаЭЃКбЁдёЬт
25ЁцЃЌ101 k PaЪБЃЌЧПЫсгыЧПМюЕФЯЁШмвКЗЂЩњжаКЭЗДгІЕФжаКЭШШЮЊ57.3 kJ/molЃЌаСЭщЕФШМЩеШШЮЊ5518 kJ/molЁЃЯТСаШШЛЏбЇЗНГЬЪНЪщаДе§ШЗЕФЪЧЃЈ ЃЉ
AЃЎ2H+(aq) +SO42Ѓ(aq)+Ba2+(aq)+2OHЃ(aq)=BaSO4(s)+2H2O(1)ЃЛІЄH=Ѓ57.3 kJ/mol
BЃЎKOH(aq)+ H2 SO4(aq)=K2SO4(aq)+H2O(l)ЃЛІЄH=Ѓ57.3kJ/mol
CЃЎ2C8H18(g)+25O2(g)=16CO2(g)+18H2O(1)ЃЛІЄH=Ѓ5518 kJ/mol
DЃЎC8H18(l)+ 25/2 O2(g)=8CO2(g)+ 9H2O(1)ЃЛІЄH=Ѓ5518 kJ/mol
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2009ИпПМецЬтЛуБр-бѕзхдЊЫиЃЌЛЗОГБЃЛЄ ЬтаЭЃКбЁдёЬт
25 ЁцЃЌ101 k PaЪБЃЌЧПЫсгыЧПМюЕФЯЁШмвКЗЂЩњжаКЭЗДгІЕФжаКЭШШЮЊ57.3 kJ/molЃЌаСЭщЕФШМЩеШШЮЊ5518 kJ/molЁЃЯТСаШШЛЏбЇЗНГЬЪНЪщаДе§ШЗЕФЪЧ
A.2H+(aq) + (aq)+
(aq)+ (aq)+2OH
(aq)+2OH (aq)=BaSO4(s)+2H
(aq)=BaSO4(s)+2H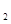 O(1);
O(1); H=
H= 57.3
kJ/mol
57.3
kJ/mol
 B.KOH(aq)+
B.KOH(aq)+ H
H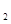 SO4(aq)=
SO4(aq)=  K
K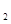 SO4(aq)+H
SO4(aq)+H O(I);
O(I);  H=
H= 57.3kJ/mol
57.3kJ/mol
C.C8H18(I)+  O
O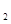 (g)=8CO
(g)=8CO (g)+ 9H
(g)+ 9H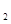 O;
O;  H=
H= 5518 kJ/mol
5518 kJ/mol
 D.2C8H18(g)+25O
D.2C8H18(g)+25O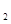 (g)=16CO
(g)=16CO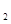 (g)+18H
(g)+18H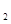 O(1);
O(1);  H=
H= 5518 kJ/mol
5518 kJ/mol
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com