


 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
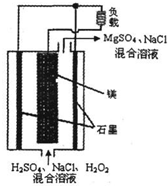 þ-��������ȼ�ϵ�ؾ��б������ߡ���ȫ������ŵ㣬��ṹʾ��ͼ��ͼ��ʾ�� ���ڸõ�ص�������ȷ���ǣ�������
þ-��������ȼ�ϵ�ؾ��б������ߡ���ȫ������ŵ㣬��ṹʾ��ͼ��ͼ��ʾ�� ���ڸõ�ص�������ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�ʹ�һ�и�һ�ڶ����¿���ѧ�Ծ����������� ���ͣ���ѡ��
��һ�������£��ֱ��Ը�����ء�����ء���������(H202)Ϊԭ����ȡ���������Ƶ�ͬ�¡�ͬѹ����ͬ�����O2ʱ��������Ӧ��ת�Ƶĵ�����֮��Ϊ ( )
| A��l ��l ��1 | B��2 ��2 ��l | C��2 ��3 ��1 | D��4 ��3 ��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ��һ�ڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ�������£��ֱ��Ը�����ء�����ء���������(H202)Ϊԭ����ȡ���������Ƶ�ͬ�¡�ͬѹ����ͬ�����O2ʱ��������Ӧ��ת�Ƶĵ�����֮��Ϊ ( )
A��l ��l ��1 B��2 ��2 ��l C��2 ��3 ��1 D��4 ��3 ��2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com