
����Ŀ��̿���������е���Ҫ������֮һ���о����������Ի���������ɻ����������Կ�����������������̵������仯ģ���������ͼ��ʾ������˵���������
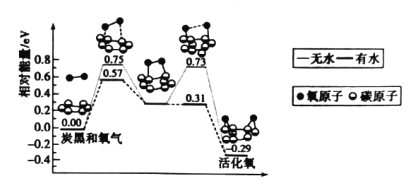
A.̿�ڿ����Ǵ����ж�������ת��Ϊ��������Ĵ���
B.�����ӵĻ����O-O���Ķ�����C-O��������
C.ˮ��ʹ�����ӻ��Ӧ�Ļ�ܽ���0.42 eV
D.ÿ�һ�������ӷų�0.29 eV������
���𰸡�C
��������
A. ������Կ���������������̿�ڿ������Ի�����ӣ����̿�ڿ������Կ��������ж�������ת��Ϊ��������Ĵ�����A��ȷ��
B������ͼ������������ӻ����O-O�����ѣ�����C-O�������������ӵĻ��O-O�Ķ�����C-O�������ɹ��̣�B��ȷ��
C�����ܷ�Ӧ�����д��ڶಽ��Ӧ�Ļ�ܣ�������Ӧ�Ļ�Ϊ��ܽϴ��ߣ���������ͼ������������Ӧ�Ļ��Ϊ��ܽϴ��ߣ���û��ˮ����ķ�Ӧ���ΪE=0.75eV����ˮ����ķ�Ӧ�Ļ��ΪE=0.57eV������ˮ��ʹ�����ӻ��Ӧ�Ļ�ܽ���0.75 eV-0.57 eV=0.18 eV��C����
D����ͼ��֪����Ӧ�������������������������������ÿ�һ�������ӷų�0.29 eV��������D��ȷ��
�ʺ���ѡ����C��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪X��Y��Z��W��R����Ԫ�ؾ�λ�����ڱ���ǰ�����ڣ���ԭ��������������Ԫ��X�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Y�Ļ�̬ԭ���е���ռ��������������ͬ��ԭ�ӹ�����������ֹ���еĵ�������ͬ��Wλ�ڵ�2���ڣ����̬ԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������3����R��̬ԭ��3d����ϵĵ�������4s����ϵ�4������ش��������⣺������ʱ��X��Y��Z��W��R������Ӧ��Ԫ�ط��ű�ʾ��
�Ż�̬Rԭ�ӵ���Χ�����Ų�ʽΪ______��Y��Z��W�ĵ縺���ɴ�С��˳����____��
��Ԫ��Y��Z������Ԫ��X�γɺ�18�����ӵ�������Щ���зе���ߵ���______����е���ߵ�ԭ����______��Y2X4������X2W��ԭ����_____��
��YW�ĽṹʽΪ______(�������е���λ��)���ڻ�ѧʽΪ��R(ZX3)4(X2W)2��2+������������R�γ���λ����ԭ����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˲ⶨ�Ҵ��Ľṹʽ���������������ˮ�ƾ����Ʒ�Ӧ��ʵ��װ�úͲⶨ���������װ�ý���ʵ�顣�ɹ�ѡ�õ�ʵ��������ͼ��ʾ��

��ش��������⣺
(1)���������������ȷװ����________(��д���)��
(2)װ����A���ֵķ�Һ©����������ƿ֮�����ӵĵ��������������________(��д���)��
A����ֹ��ˮ�ƾ��ӷ�
B����֤ʵ��װ�ò�©��
C��ʹ��ˮ�ƾ�������
(3)ʵ��ǰԤ�Ƚ�С�����ڶ��ױ����ۻ���С���飬��ȴ������ƿ�У���Ŀ����
________________________________________________________________________��
(4)��֪��ˮ�ƾ����ܶ�Ϊ0.789 g��cm��3����ȡ2.0 mL�ƾ�����Ӧ��ȫ��(�ƹ���)���ռ�390 mL���塣���Ҵ��������ܱ���ȡ��������ԭ����Ϊ________���ɴ˿�ȷ���Ҵ��ĽṹʽΪ________________������____________________________________________________��
(5)ʵ�����ⶨ�Ľ��ƫ�ߣ����������ԭ����________(��д���)��
A����ʵ���������½���
B����ˮ�ƾ��л������״�
C����ˮ�ƾ����Ƶķ�Ӧ������ȫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪CrO42-��Cr2O72-����Һ�п��ת���������£���ʼŨ��Ϊ1.0 mol��L��1��Na2CrO4��Һ��c(Cr2O72-)��c(H��)�ı仯��ͼ��ʾ��
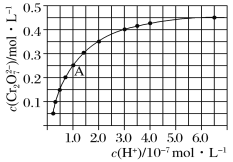
(1)�����ӷ���ʽ��ʾNa2CrO4��Һ�е�ת����Ӧ______��
(2)��ͼ��֪����Һ��������CrO42-��ƽ��ת����____(�������С�����䡱)������A�����ݣ��������ת����Ӧ��ƽ�ⳣ��Ϊ________��
(3)�����¶ȣ���Һ��CrO42-��ƽ��ת���ʼ�С����÷�Ӧ����H________0(����ڡ���С�ڡ����ڡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ����Ҫ�����ʣ���������ȡ���ʺ�����ȡ���֪H-H����N-H����N��N���ļ��ֱܷ���436kJ/mol��391kJ/mol��946kJ/mol��
(1)д���ϳɰ����ȷ�Ӧ����ʽ__________������1 mol NH3��Ҫ���ջ�ų�_______ kJ��������
(2)���ϳɰ���Ӧ�ﵽƽ��ı�ijһ�������![]() ���ı�N2��H2��NH3����
���ı�N2��H2��NH3����![]() ����Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾͼ��t1ʱ����ƽ���ƶ�������������_______�����б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����________��
����Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾͼ��t1ʱ����ƽ���ƶ�������������_______�����б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����________��
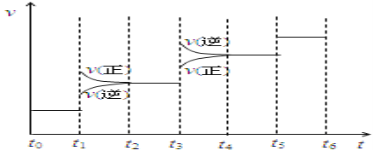
(3)�¶�ΪT0Cʱ����2a molH2��a molN2����0.5L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50%����÷�Ӧ��ƽ�ⳣ��Ϊ_________��
(4)��֪373Kʱ�����淴Ӧƽ�ⳣ��ΪK=0.12����ijʱ��ʱ�����c(N2)=1 mol/L��c(H2)=3 mol/L��c(NH3)=2 mol/L����ʱ���淴Ӧ_________��
A.����������� B.���淽����� C����ƽ��״̬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԥ�⣬��2040���ҹ�ú̿�����Խ�ռ��Դ�ṹ������֮һ���ҡ�H2S�ڴ�����̼��AC�������Ǩ�ƣ���ú�������ۺ�Ӧ�����˺ܴ�Ĵٽ����ã��������ͼ��ʾ������ad��ʾ���ֵ�����״̬�������й������������

A.ͼ����Ӱ���ֱ�ʾH2S���ӵ����������
B.AC�������õ��¶Ȳ�ͬ��H2S��ȥ���ʲ�ͬ
C.H2S��AC�����������ɵIJ�����H2O��H2��S��SO2��CS2��
D.ͼ�з�Ӧ������ֻ��H��S���Ķ��ѣ�û��H��S�����γ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ҽ�÷������ֵ�ԭ�Ͼ۱�ϩ��ά�����л��߷��Ӳ��ϣ��䵥��Ϊ��ϩ����ϩ���˺ϳɾ۱�ϩ�⣬���㷺�����Ʊ�1��2�����ȱ��顢��ϩȩ����ϩ��ȡ���ش��������⣺
��.��ҵ���ñ�ϩ�ӳɷ��Ʊ�1��2�����ȱ��飬��Ҫ������Ϊ3���ȱ�ϩ����Ӧԭ��Ϊ��
��![]()
![]()
��![]()
![]()
��1����֪![]() �Ļ��
�Ļ��![]() ���棩Ϊ
���棩Ϊ![]() ����÷�Ӧ��
����÷�Ӧ��![]() ���������Ϊ_____
���������Ϊ_____![]() ��
��
��2��һ���¶��£�������ܱ������г�������ʵ�����![]() ��
��![]() ���ڴ��������·�����Ӧ�٢ڣ������������ѹǿ��ʱ��ı仯���±���ʾ��
���ڴ��������·�����Ӧ�٢ڣ������������ѹǿ��ʱ��ı仯���±���ʾ��
ʱ��/min | 0 | 60 | 120 | 180 | 240 | 300 | 360 |
ѹǿ/kPa | 80 | 74.2 | 69.4 | 65.2 | 61.6 | 57.6 | 57.6 |
�õ�λʱ���������ѹ�ı仯����ʾ��Ӧ���ʣ���![]() ����Ӧ��ǰ180min��ƽ����Ӧ����
����Ӧ��ǰ180min��ƽ����Ӧ����![]() ____
____![]() ������С�����2λ����
������С�����2λ����
��.��ϩ���Ʊ�����
����һ�������������ⷨ�Ʊ���ϩ��Ӧ���£�
![]()
![]()
��3����ij�¶��£��ڸ��������г���![]() ����ʼѹǿΪ10kPa��ƽ��ʱ��ѹΪ14kPa��
����ʼѹǿΪ10kPa��ƽ��ʱ��ѹΪ14kPa��![]() ��ƽ��ת����Ϊ______���÷�Ӧ��ƽ�ⳣ��
��ƽ��ת����Ϊ______���÷�Ӧ��ƽ�ⳣ��![]() ______kPa������С�����2λ����
______kPa������С�����2λ����
����ѹ�ֱ�Ϊ100kPa��10kPaʱ�����÷�Ӧ��ƽ����ϵ��![]() ��
��![]() �����ʵ����������¶ȱ仯��ϵ��ͼ��ʾ��
�����ʵ����������¶ȱ仯��ϵ��ͼ��ʾ��
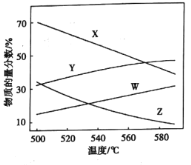
10kPaʱ��![]() ��
��![]() �����ʵ����������¶ȱ仯��ϵ�����߷ֱ���_____��______��
�����ʵ����������¶ȱ仯��ϵ�����߷ֱ���_____��______��
�������������������ⷨ�Ʊ���ϩ������![]() ��
��![]() �ȸ�����Ʊ���ϩ�ķ�Ӧ���£�
�ȸ�����Ʊ���ϩ�ķ�Ӧ���£�
![]()
![]() ���ڴ���������
���ڴ���������![]() ��ת���ʺ�
��ת���ʺ�![]() �IJ������¶ȱ仯��ϵ��ͼ��ʾ��
�IJ������¶ȱ仯��ϵ��ͼ��ʾ��

��4����ͼ��![]() ��ת�������¶����߶�������ԭ����_________��
��ת�������¶����߶�������ԭ����_________��
��575��ʱ��![]() ��ѡ����Ϊ___����
��ѡ����Ϊ___����![]() ��ѡ����=
��ѡ����=![]() ��
��
�ۻ��������о�����������![]() ѡ���ԵĴ�ʩ��______��
ѡ���ԵĴ�ʩ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������X����ij�ܱ������У�������Ӧ��2X(g)![]() 3Y(g)+Z(g)�����������X�����ʵ����������¶ȹ�ϵ��ͼ��ʾ�������ƶ���ȷ����()��
3Y(g)+Z(g)�����������X�����ʵ����������¶ȹ�ϵ��ͼ��ʾ�������ƶ���ȷ����()��
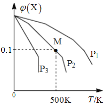
A. �����¶ȣ��÷�Ӧƽ�ⳣ��K��С
B. ѹǿ��С��P3��P2��P1
C. ƽ�������Ч����ʹƽ��Ħ����������
D. �ڸ�������M��Xƽ��ת����Ϊ9/11
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���廯����һ����Ҫ���廯��������������ֽ��������������ȣ���ʯ���顢Һ�弰����Ϊԭ���Ʊ�CaBr22H2O��ʵ���������£�
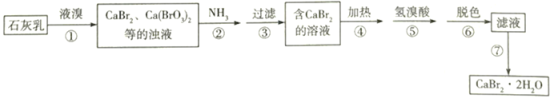
(1)�������������70�����£��¶Ȳ��˹��ߵ�ԭ����________________��
(2)����ʵ������ȡ����NH3�ķ�����ȷ����_____________�����ţ���
A�� B��
B�� C��
C�� D��
D��
(3)��֪NH3������ΪN2��������з�����Ӧ�Ļ�ѧ����ʽΪ_______________________��
(4)����ܡ��ݵ�Ŀ��������_______________________________��________________________��
(5)�������_________________������ɫ���������˸����ʵ�____________________��
(6)����ߵõ���Ʒ�IJ���������__________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com