
A��B��C��DΪ��ѧ��ѧ�г��������ֵ��ʡ�
��1����֪�ڿ�����AԼռ21%��Ϊ�����������C������CS2�������ڿ�������ȼ��D
�ڱ�״���µ��ܶ�Ϊ3.170 g?L��1���ֱ�д��A��C��D�Ļ�ѧʽ��A ��C ��D ����ҵ����ȡC��������ķ�Ӧ��ѧ����ʽΪ��
��2����һ��������B��A��C��D���Ϸֱ����ɼס��ҡ�������֪�ҡ��������к��еĵ���������K+��ͬ���ҵĻ�ѧʽ�ǣ��� �����ĵ���ʽ�ǣ� ��
��3��C���Է�������ͼ��ʾһϵ��ת���������ʼ���Ӧ�������ԣ�

�����ĵ���A�뻯�����ҷ�Ӧ�Ļ�ѧ����ʽ ��Z��Ũ��Һ��ͭ���ڼ��ȵ������·�Ӧ������Ӧ����1.806��1024������ת��ʱ������ԭ��Z���ʵ����� mol��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
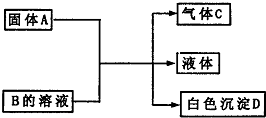 A��B��C��DΪ��ѧ��ѧ���������ʣ����Ǽ�ķ�Ӧ��ϵ��ͼ��ʾ��
A��B��C��DΪ��ѧ��ѧ���������ʣ����Ǽ�ķ�Ӧ��ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
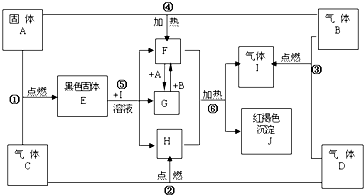
 ��ͼ��ʾ��A��B��C��DΪ��ѧ��ѧ�������ʻ��������Ӧ��Դ����ѧ��ѧ�̲ģ���Ҫ����գ�
��ͼ��ʾ��A��B��C��DΪ��ѧ��ѧ�������ʻ��������Ӧ��Դ����ѧ��ѧ�̲ģ���Ҫ����գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������֣�����A��B��C��DΪ��ѧ�������ʣ������Ԫ�ص�ԭ���������μ�С��������ֻ�������Ƕ�����Ԫ�أ�BΪ����ɫ���塣�Ը�����ͼ�����ʵ��ת����ϵ�ش��������⡣
��1��A��_________
��2������ת���У�A�������� ���ڢ١��ķ�Ӧ�У�������������ԭ��Ӧ���� ��������������֣�
��3��д��A�ڸ�������H��Ӧ�Ļ�ѧ��Ӧ����ʽ���������ת�Ƶķ������Ŀ
���������� ��
��4��д����Ӧ�Ļ�ѧ��Ӧ����ʽ���������� ��
��5����֪��101 kPa �����£�2 mol D��ȫȼ������Һ̬������ų����������� kJ����������д��ʾDȼ���ȵ��Ȼ�ѧ����ʽ�� ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�콭��ʡӥ̶�и����ڶ���ģ�⿼�����ۻ�ѧ���� ���ͣ������
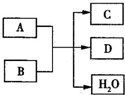
�������֣�����A��B��C��DΪ��ѧ�������ʣ������Ԫ�ص�ԭ���������μ�С��������ֻ�������Ƕ�����Ԫ�أ�BΪ����ɫ���塣�Ը�����ͼ�����ʵ��ת����ϵ�ش��������⡣
��1��A��_________
��2���� ��ת���У�A�������� ���ڢ١��ķ�Ӧ�У�����������
��ת���У�A�������� ���ڢ١��ķ�Ӧ�У����������� ��ԭ��Ӧ���� ��������������֣�
��ԭ��Ӧ���� ��������������֣�
��3��д��A�ڸ�������H��Ӧ�Ļ�ѧ��Ӧ����ʽ���������ת�Ƶķ������Ŀ
���������� ��
��4��д����Ӧ�Ļ�ѧ��Ӧ����ʽ���������� ��
��5����֪��101 kPa �����£�2 mol D��ȫȼ������Һ̬������ų����������� kJ����������д��ʾDȼ���ȵ��Ȼ�ѧ����ʽ������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com