
п�̵�أ��׳Ƹɵ�أ��������е������ܴ�����п�̵�صĹ���ͼ��ͼ��a����ʾ��
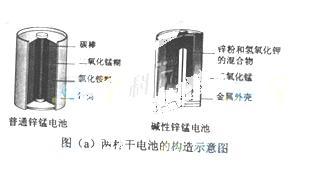
�ش��������⣺
��1������ͨп�̵�طŵ�ʱ��������Ҫ��ӦΪ��Zn+2NH4Cl+2MnO2=Zn(NH3)2Cl2+2MnOOH���õ���У�����������Ҫ��___ _________________������ʵ���Ҫ�ɷ���__________��������������Ҫ��Ӧ��________________________________________________________��
_________________������ʵ���Ҫ�ɷ���__________��������������Ҫ��Ӧ��________________________________________________________��
������ͨп�̵����ȣ�����п�̵�ص��ŵ㼰��������_______��
��2��ͼ��b����ʾ�������÷Ͼ���ͨп�̵�ص�һ�ֹ��գ������ǷϾɵ����ʵ�ʴ��ڵ�����������������

��ͼ��b���в���Ļ�ѧʽ�ֱ�ΪA_______��B________��
�ڲ���a�еõ��ۿ����Ҫ�ɷ���K2MnO4������b�У���ɫ��K2MnO4��Һ��Ӧ��������ɫ��Һ��һ�ֺں�ɫ���壬�÷�Ӧ�����ӷ���ʽΪ_______��
�۲��ö��Ե缫���K2MnO4��ҺҲ�ܵõ�������D�����������õ�����Ҫ������____�����ѧʽ��
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ����٤��������ֵ������˵����ȷ����
A�������£�0.2molFe������ˮ������Ӧ�����ɵ�H2������ĿΪ0.3NA
B�������£�1LpH��13��NaOH ��Һ�У���ˮ�����OH��������ĿΪ0.1NA
��Һ�У���ˮ�����OH��������ĿΪ0.1NA
C������ȼ�ϵ����������22.4L����״��������ʱ����·��ͨ���ĵ�����ĿΪ2NA
D��5NH4NO3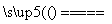 2HNO3��4N2����9H2O��Ӧ�У�����28g N2ʱ��ת�Ƶĵ�����ĿΪ3.75NA
2HNO3��4N2����9H2O��Ӧ�У�����28g N2ʱ��ת�Ƶĵ�����ĿΪ3.75NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������ѧϰ��ѧ����Ҫ���ߡ�����������ʾ���ʱ仯�Ļ�ѧ�����У��������
A��������ʴʱ���ܷ�����������Ӧ��2H2O + O2 + 4e- = 4OH-
B����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽ��2H2��g��+O2��g��=2H2O��g������H=-483.6kJ/mol
C������ˮ������ӷ���ʽ��CO32- + H2O  HCO3- + OH-
HCO3- + OH-
D��Ca(HCO3)2��Һ�м����������ʯ��ˮ��Ca2++HCO3��+OH��=CaCO3��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ε�ⷨ��õĴ�������һ�����Ľ����ƺ���������Щ���ʿɲ��ô�����������ȥ��������β������������øֲĶ����������������£�
(ע��NaCl�۵�Ϊ801 �棻AlCl3��181 ������)
(1)����ǰ������������������������ʯӢɰ����ֹ����ʱ���Ƿֱ����������û���Ӧ�����µ����ʣ���صĻ�ѧ����ʽΪ��_____________��
��___________________��
(2)��Cl2����ͨ�������еĴ������壬�����������ϸ���ȥ�����ݵ���Ҫ�ɷֳ�Cl2�����_____________��
��̬����ճ���������ϣ�����������γɸ����������п϶�����________________��
(3)���÷ϼ�Һ����A�Ĺ����У���������Ӧ�����ӷ���ʽΪ_____________��
(4)�ֲĶ��������γɵ�����������Ĥ�ܷ�ֹ�ֲĸ�ʴ����ԭ����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
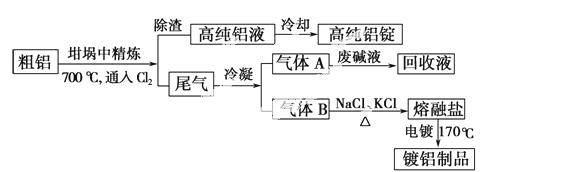
��ʯ��Ҫ������ơ�Ca3(PO4)2·H2O������ʯ��Ca5(OH)(PO4)3������ʽ���ڡ�ͼ(a)ΪĿǰ��������ʯ���õĴ������������ʪ��������ָ��ʯ�ù�������ֽ��Ʊ����ᡣͼ(b)���ȷ�������������������ʯ�Ƶ��������̡�
�������ʵ�����������£�
| �۵�/�� | �е�/�� | ��ע | |
| ���� | 44 | 280.5 | |
| PH3 | -133.8 | -87.8 | ������ˮ�����л�ԭ�� |
| SiF4 | -90 | -86 | ��ˮ�� |
�ش��������⣺
��1����������ʯ����Ҫ����;�����������ϣ�Լռ��ʯʹ������ ℅��
��2������ʯΪԭ�ϣ�ʪ�����������Ca5F(PO4)3��Ӧ�Ļ�ѧ����ʽΪ��  ������1���ۺϺ�������������Լ30%����ʯ�������Ƶ�85℅����Ʒ
������1���ۺϺ�������������Լ30%����ʯ�������Ƶ�85℅����Ʒ ���� �֡�
���� �֡�
��3����ͼ(b)��ʾ���ȷ���������ĵ�һ���ǽ��������衢������̿����ʯ��ϣ����·�Ӧ���ɰ��ס�¯������Ҫ�ɷ��ǣ� (�ѧʽ)������1����Ҫ�������ǣ� ������2����Ҫ�������ǣ�
��4��β������Ҫ���� ������������PH3��H2S��HF�ȣ���β ����ͨ�봿����Һ��
����ͨ�봿����Һ�� �ɳ�ȥ
�ɳ�ȥ 
��ͨ�����������Һ���ɳ�ȥ (���ѧʽ)
��5�������ʪ�����ᣬ�ȷ����Ṥ�ո��ӣ��ܺĸߣ����ŵ��ǣ�  ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ���������������ͼ���£�

��ش��������⣺
(1)�������������Ի�����Ϊԭ�ϣ������ڹ����������������Ϊԭ�ϣ�������________________________________________________________________________��
(2)������������Ӧ��ǰ�辻����ԭ����_________________________ ________
________
_____________________ ___________________________________________________��
___________________________________________________��
(3)�ڴ���Ӧ����ͨ��ʹ�ó�ѹ���ڴ�������SO2��ת����Ϊ90%�����Dz��ַ�����Ҳ�ȡ��ѹ��������ȡSO3����ȡ��ѹ��ʩ��Ŀ�ij��˼ӿ췴Ӧ�����⣬������____________________________���Ӷ��������Ч�ʡ�
(4)��ҵ�����г��ð�—�ᷨ����β�������Դﵽ������Ⱦ���������õ�Ŀ�ġ��û�ѧ����ʽ��ʾ�䷴Ӧԭ����____________________________________________________
________________________________________________________________________
________________________________________________________________________��
(5)�����Ṥҵ�⣬�������ҵ������������صĹ�ҵ������������ȷ����________��
A����ˮ���壺��ˮŨ���ȿ�����ˮ������������Һ����Һ��
B����ˮ��þ����̲����ʯ��ˮMgO�ۻ����þ
C����ҵ���������NO2ˮ�������ᡪ��β������
D����ҵ�ϳɰ�����Ȼ��һ�������������ϳ�����������NH3��H2��N2ˮ����백
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ�������ʾ��ͼ���£�
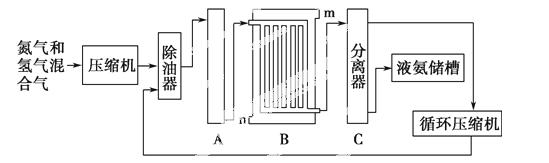
�ش��������⣺
(1)��ҵ�ϳɰ���ԭ���ǵ����������������Ǵӿ����з�������ģ�ͨ��ʹ�õ����ַ��뷽����________��________����������Դ��ˮ��̼�⻯���д���ֱ����ú����Ȼ��Ϊԭ����ȡ�����Ļ�ѧ����ʽ_______________________��
(2)�豸A�к��е����������ý���Ƚ��������豸A��������________�����з����Ļ�ѧ��Ӧ����ʽΪ____________��
(3)�豸B������Ϊ________������m��n������ͨˮ�ڣ���ˮ����________(�m����n��)�����˴��෴����ͨˮ��ԭ����______________��
(4)�豸C��������______________________��
(5)��ԭ�����Ʊ������л��е�CO�Դ����ж������ã�����ȥԭ�����е�CO����ͨ�����·�Ӧ��ʵ�֣�
CO(g)��H2O(g)  CO2(g)��H2(g)
CO2(g)��H2(g)
��֪1 000 Kʱ�÷�Ӧ��ƽ�ⳣ��K��0.627����ҪʹCO��ת���ʳ���90%������ʼ����c(H2O)��c(CO)������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ����������Ĺؼ�����ѧΪ����������������ṩ�����ʱ�֤��
(1)���ʱ���öƲ������������������____________________________________��Ϊ��ʹ�Ʋ��Ⱦ��ȡ��⻬���ܡ���Ƽ��ĸ�����22____��
(2)±ˮ���̺��ŷḻ��þ��Դ����ת����ɻ��MgCl2�ֲ�Ʒ����±ˮ����ȡþ�IJ���Ϊ
a�������ߴ������ڵı������ճ�ʯ�ң�����ʯ���Ƴ�ʯ���飻
b����ʯ������뵽��ˮ�������о����˵õ�Mg(OH)2������
c����Mg(OH)2�����м�������õ�MgCl2��Һ���پ������ᾧ�õ�MgCl2·6H2O��
d����MgCl2·6H2O��һ�������¼��ȵõ���ˮMgCl2��
e��������ڵ��Ȼ�þ�ɵõ�Mg��
�ٲ���d�еġ�һ��������ָ����__________________��Ŀ����_________________��
��������ȡþ�������У�Ϊ�˽��ͳɱ���������Ⱦ�����Բ�ȡ�ܶ��ʩ����д������һ��________________________________________________________________________��
����ͬѧ��Ϊ������b��ɼ���Mg(OH)2�õ�MgO���ٵ�����ڵ�MgO�ƽ���þ�������ɼ�ʵ�鲽�裬��ͬ���ͬѧ���뷨��Ϊʲô��
(3)���Ǻ˷�Ӧ����Ҫ��ȼ�ϣ��Ѿ����Ƴɹ�һ���������ӽ�����֬����ר��������ˮ�е�U4����������������Ԫ�ء��䷴Ӧԭ��Ϊ____________________________________
________________________________________________________________________(��֬��HR����)��
�������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ��Ϊ________________________________________________________________________
________________________________________________________________________��
(4)��˾ƥ��(COOHOOCCH3) �ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��______________
�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��______________
________________________________________________________________________��
�˷�Ӧ����������________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��2Fe + 3Br2 = 2FeBr3��Fe2+�Ļ�ԭ�Դ���Br��������16.8 g����0.3 mol Br2��Ӧ�����ˮ�õ�������Һ��ͨ��a mol Cl2��������������ȷ����
A����a = 0.1ʱ�������ķ�ӦΪ2Fe2++Cl2��2Fe3++2Cl��
B����a = 0.45ʱ�������ķ�ӦΪ2Fe2++4Br��+3Cl2��2Fe3++2Br2+6Cl��
C������Һ��Br����һ�뱻����ʱ�� c(Fe3+): c(Br��):c(Cl��) ��1:1:3
D����0��a��0.15ʱ����Һ��ʼ������2c(Fe2+)+3c(Fe3+)+c(H+)��c(Cl��)+c(Br��)+ c(OH��)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com