
 ÷–Α¥ΧεΜΐ±»1ΓΟ4ΓΟ4ΒΡ±»άΐ≈δ÷Τ≈®ΝρΥαΓΔ““¥ΦΚΆ““ΥαΒΡΜλΚœ»ή“ΚΓΘ
÷–Α¥ΧεΜΐ±»1ΓΟ4ΓΟ4ΒΡ±»άΐ≈δ÷Τ≈®ΝρΥαΓΔ““¥ΦΚΆ““ΥαΒΡΜλΚœ»ή“ΚΓΘ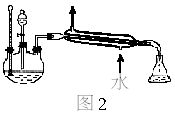
ΦϊΫβΈω


 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ‘ΡΕΝάμΫβ
| Έο÷ | »έΒψΘ®ΓφΘ© | Ζ–ΒψΘ®ΓφΘ© | ΟήΕ»Θ®g/cm3Θ© |
| ““¥Φ | -117.0 | 78.0 | 0.79 |
| ““Υα | 16.6 | 117.9 | 1.05 |
| ““Υα““θΞ | -83.6 | 77.5 | 0.90 |
| ≈®ΝρΥαΘ®98%Θ© | - | 338.0 | 1.84 |
| ≈®ΝρΥα |
| Φ”»» |
| ≈®ΝρΥα |
| Φ”»» |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚΗΏ÷–Μ·―ßœΑΧβ Χβ–ΆΘΚ058
“―÷Σœ¬Ν– ΐΨίΘΚ
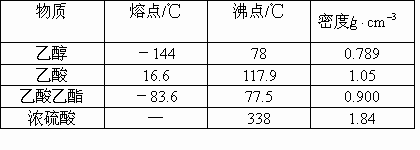
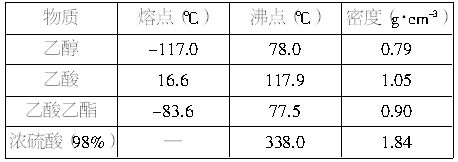
Β―ι “÷Τ““Υα““θΞΒΡ÷ς“Σ≤Ϋ÷ηΘΚ
‘Ύ30mLΒΡ¥σ ‘Ιή÷–Α¥1ΘΚ4ΘΚ4ΒΡ±»άΐ≈δ÷Τ≈®![]() ΓΔ““¥ΦΚΆ““ΥαΒΡΜλΚœ“ΚΘ°
ΓΔ““¥ΦΚΆ““ΥαΒΡΜλΚœ“ΚΘ°
ΔΎ»γΆΦ5-22Ν§Ϋ”ΉΑ÷ΟΘ§ Ι≤ζ…ζΒΡ’τΤχΨ≠ΒΦΙήΆ®ΒΫ Δ”–4mL±ΞΚΆ![]() »ή“Κ(Φ”»κΒΈΖ”ΧΣ ‘“ΚΒΡ15mL ‘Ιή÷–Θ°)
»ή“Κ(Φ”»κΒΈΖ”ΧΣ ‘“ΚΒΡ15mL ‘Ιή÷–Θ°)
Δέ–ΓΜπΦ”»»¥σ ‘Ιή÷–ΒΡΜλΚœΈο
Δή¥ΐ–Γ ‘Ιή ’Φ·ΒΫ‘Φ4mL≤ζΈο ±ΆΘ÷ΙΦ”»»Θ§≥Ζ≥ω–Γ ‘Ιή≤Δ”ΟΝΠ’ώΒ¥Θ§»ΜΚσΨ≤÷Ο¥ΐΤδΖ÷≤ψΘ°
ΔίΖ÷άκ¥ΩΨΜΒΡ““Υα““θΞ
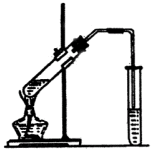
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)»γΚΈ≈δ÷Τ’β“ΜΕ®±»άΐΒΡΜλΚœ“Κ________Θ°
(2)–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ________Ζ¥”Π÷–≈®![]() ΒΡΉς”Ο «________Θ°
ΒΡΉς”Ο «________Θ°
(3)‘ΎΖ¥”ΠΉΑ÷Ο÷–Θ§ΒΦΤχΙή≥ωΩΎΒΡΈΜ÷Ο‘Ύ![]() “ΚΟφ…œΖΫ2Θ≠3mm¥ΠΘ§Τδάμ”… «________ΘΜ≤Ϋ÷ηΔέ÷–ΈΣ ≤Ο¥“Σ–ΓΜπΦ”»»________Θ°
“ΚΟφ…œΖΫ2Θ≠3mm¥ΠΘ§Τδάμ”… «________ΘΜ≤Ϋ÷ηΔέ÷–ΈΣ ≤Ο¥“Σ–ΓΜπΦ”»»________Θ°
(4)÷Η≥ω≤Ϋ÷ηΔήΥυΙέ≤λΒΫΒΡœ÷œσ «________Θ°
‘≠“ρ”κΫα¬έ «________Θ°
(5)ΈΣΝΥΧαΗΏ““Υα““θΞΒΡ≤ζ¬ Θ§ΦΉΓΔ““ΝΫΆ§―ßΖ÷±π…ηΦΤΝΥ»γΆΦ5-23ΓΔΆΦ5-24ΉΑ÷ΟΘ§““Ά§―ߥΐΖ¥”ΠΆξ±œΚσΘ§Ζ≈άδ…’ΤΩ÷–ΒΡ“ΚΧεΘ§‘Ό”Ο±ΞΚΆ![]() »ή“ΚΧα»Γ≤ζΈοΘ§Ρψ»œΈΣΡΡ÷÷ΉΑ÷ΟΚœάμΘΩΈΣ ≤Ο¥ΘΩ
»ή“ΚΧα»Γ≤ζΈοΘ§Ρψ»œΈΣΡΡ÷÷ΉΑ÷ΟΚœάμΘΩΈΣ ≤Ο¥ΘΩ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013ΫλΡΎΟ…Ι≈ΑΆ –ΗΏΕΰœ¬―ßΤΎ4‘¬‘¬ΩΦΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ Β―ιΧβ
“―÷Σœ¬Ν– ΐΨίΘΚ

œ¬ΆΦΈΣ Β―ι “÷Τ»Γ““Υα““θΞΒΡΉΑ÷ΟΆΦΓΘ

(1)Β±±ΞΚΆΧΦΥαΡΤ»ή“Κ…œΖΫ ’Φ·ΒΫΫœΕύ“ΚΧε ±Θ§ΆΘ÷ΙΦ”»»Θ§»Γœ¬–Γ ‘ΙήBΘ§≥δΖ÷’ώΒ¥Θ§Ψ≤÷ΟΓΘ’ώΒ¥«ΑΚσΒΡ Β―ιœ÷œσ_________(Χν―Γœν)ΓΘ
A.…œ≤ψ“ΚΧε±δ±Γ B.œ¬≤ψ“ΚΧεΚλ…Ϊ±δ«≥Μρ±δΈΣΈό…Ϊ
C.”–ΤχΧε≤ζ…ζ D.”–ΙϊœψΈΕ
(2)ΈΣΖ÷άκ““Υα““θΞΓΔ““¥ΦΓΔ““ΥαΒΡΜλΚœΈοΘ§Ω…Α¥œ¬Ν–≤Ϋ÷ηΫχ––Ζ÷άκΘΚ

ΔΌ ‘ΦΝ1ΉνΚΟ―Γ”Ο___________ΘΜ
ΔΎ≤ΌΉς1 «_____Θ§Υυ”ΟΒΡ÷ς“Σ“«ΤςΟϊ≥Τ «________ΘΜ
Δέ ‘ΦΝ2ΉνΚΟ―Γ”Ο___________ΘΜ
Δή≤ΌΉς2 «___________ΘΜ
Δί≤ΌΉς3÷–Έ¬Ε»ΦΤΥ°“χ«ρΒΡΈΜ÷Ο”ΠΈΣ»γœ¬ΆΦ÷–______(ΧνaΓΔbΓΔcΓΔd)Υυ ΨΘ§‘ΎΗΟ≤ΌΉς÷–Θ§≥ΐ’τΝσ…’ΤΩΓΔΈ¬Ε»ΦΤΆβΘ§ΜΙ–η“ΣΒΡ≤ΘΝß“«Τς”–_______ΓΔ_______ΓΔ_______ΓΔ______Θ§ ’Φ·““ΥαΒΡ “ΥΈ¬Ε» «___________ΓΘ

≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ0117 Ά§≤ΫΧβ Χβ–ΆΘΚ Β―ιΧβ



≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com