
ij»ÆѧŠĖȤŠ”×éĄūÓĆ·ĻĢśŠ¼ÖĘČ”FeCl3•6H2O¾§Ģ唣Ö÷ŅŖ²Ł×÷Į÷³ĢČēĻĀ£ŗ
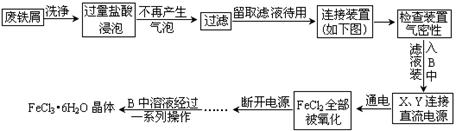
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)CÉÕ±ÖŠŹ¢·ÅµÄŹŌ¼ĮŹĒ ”£
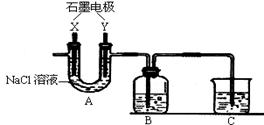
(2)AÖŠµÄX¼«Ó¦øĆĮ¬½ÓµēŌ“µÄ ¼«£¬AÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
ӣ
(3)ŹŌ¼ĮĘæBÖŠµÄČÜŅŗ¾¹żŅ»ĻµĮŠ²Ł×÷µĆµ½FeCl3•6H2O¾§Ģ壬ÕāŅ»ĻµĮŠ²Ł×÷ÓÉĻĀĮŠ²Ł×÷×é³É(²Ł×÷²»ÖŲø“)£¬ŌņŅĄ“Ī½ųŠŠµÄ²Ł×÷ĪŖ (Ģī×ÖÄøŠņŗÅ)
A£®¼ÓČČÅØĖõ B£®Ļ“µÓøÉŌļ C£®¹żĀĖ D£®ĄäČ“½į¾§
(4)øĆ×°ÖĆÓŠĪŽČ±ĻŻ£¬ČōÓŠ£¬ĒėÖø³öĄ“ ČōĪŽ£¬±¾æÕæɲ»“š”£
 »ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
»ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»ÆѧŠĖȤŠ”×éĪŖĢ½¾æĶøśÅØĮņĖįµÄ·“Ó¦£¬ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ½ųŠŠÓŠ¹ŲŹµŃ锣
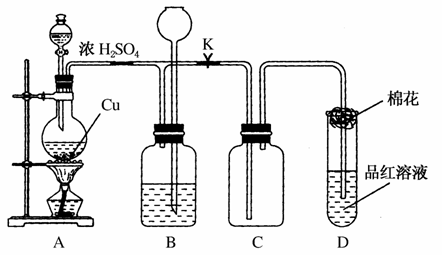
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)×°ÖĆAÖŠ·¢ÉśµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ_____________________”£
(2)×°ÖĆDÖŠŹŌ¹ÜæŚ·ÅÖƵÄĆŽ»ØÓ¦½žŅ»ÖÖŅŗĢ壬ÕāÖÖŅŗĢåŹĒ________£¬Ęä×÷ÓĆŹĒ____________________________________”£
(3)×°ÖĆBµÄ×÷ÓĆŹĒÖü“궹ӹµÄĘųĢ唣µ±D“¦ÓŠĆ÷ĻŌµÄĻÖĻóŗ󣬹Ų±Õ»īČūK£¬ŅĘČ„¾Ę¾«µĘ£¬µ«ÓÉÓŚÓąČȵÄ×÷ÓĆ£¬A“¦ČŌÓŠĘųĢå²śÉś£¬“ĖŹ±BÖŠĻÖĻóŹĒ__________________________________”£BÖŠÓ¦·ÅÖƵÄŅŗĢåŹĒ________(ĢīŃ”Ļī×ÖÄø)”£
a£®Ė® b£®ĖįŠŌKMnO4ČÜŅŗ
c£®ÅØäåĖ® d£®±„ŗĶNaHSO3ČÜŅŗ
(4)ŹµŃéÖŠ£¬Č”Ņ»¶ØÖŹĮæµÄĶʬŗĶŅ»¶ØĢå»ż18 mol”¤L£1µÄÅØĮņĖį·ÅŌŚŌ²µ×ÉÕĘæÖŠ¹²ČČ£¬Ö±µ½·“Ó¦Ķź±Ļ£¬·¢ĻÖÉÕĘæÖŠ»¹ÓŠĶʬŹ£Óą£¬øĆŠ”×éѧɜøł¾ŻĖłŃ§µÄ»ÆѧÖŖŹ¶ČĻĪŖ»¹ÓŠŅ»¶ØĮæµÄĮņĖįŹ£Óą”£
¢ŁÓŠŅ»¶ØĮæµÄÓąĖįµ«Ī“ÄÜŹ¹ĶʬĶźČ«Čܽā£¬ÄćČĻĪŖŌŅņŹĒ_________________________________________________________”£
¢ŚĻĀĮŠŅ©Ę·ÖŠÄÜÓĆĄ“Ö¤Ć÷·“Ó¦½įŹųŗóµÄÉÕĘæÖŠČ·ÓŠÓąĖįµÄŹĒ________(ĢīŃ”Ļī×ÖÄø)”£
a£®Ģś·Ū b£®BaCl2ČÜŅŗ
c£®Ņų d£®Na2CO3ČÜŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
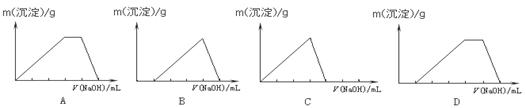
ŌŚAl2(SO4)3ŗĶ(NH4)2SO4µÄ»ģŗĻČÜŅŗÖŠ£¬ÖšµĪ¼ÓČėNaOHČÜŅŗÖĮ¹żĮ攣ĻĀĮŠĶ¼Ź¾ÄÜÕżČ·±ķŹ¾Éś³É³ĮµķµÄÖŹĮæÓėµĪČėNaOHČÜŅŗĢå»ż¹ŲĻµµÄŹĒ£Ø £©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÉčNAĪŖ°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ£Ø £©
A£®1molĶÓė×ćĮæµÄĮņ·“Ó¦Ź±Ź§Č„µÄµē×ÓŹżĪŖNA
B£®7.8g Na2SŗĶNa2O2µÄ»ģŗĻĪļÖŠŗ¬ÓŠµÄŅõĄė×ÓŹż“óÓŚ0.1 NA
C£®0.5 mol NO2ĘųĢå½µĪĀŗóŃÕÉ«±äĒ³£¬ĘäĖłŗ¬µÄ·Ö×ÓŹżČŌČ»ĪŖ0.5 NA
D£®1molFeCl3ŗĶ·ŠĖ®ĶźČ«·“Ó¦×Ŗ»ÆĪŖĒāŃõ»ÆĢś½ŗĢåŗó£¬ĘäÖŠ½ŗĢåĮ£×ÓŹżÄæĪŖNA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĄūÓĆÉś»īÖŠ³£¼ūµÄ²ÄĮĻæÉŅŌ½ųŠŠŗܶąæĘѧŹµŃ飬ĻĀĶ¼¾ĶŹĒŅ»øöÓĆ·Ļ¾É²ÄĮĻÖĘ×÷µÄæÉÓĆÓŚĒż¶ÆĶę¾ßµÄµē³ŲŹ¾ŅāĶ¼”£µ±µē³Ų¹¤×÷Ź±£¬ÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ£Ø £©
A£®ĀĮ¹Ž½«Öš½„±»øÆŹ“
B£®øƵē³ŲĒż¶Æµē¶ÆĶę¾ßŹ±,ŹÆÄ«°ōÓ¦ÓėĶę¾ßµē»śµÄøŗ¼«ĻąĮ¬
C£®ŹÆÄ«°ōÉĻ·¢ÉśµÄ·“Ó¦ĪŖ£ŗO2£4e££«2H2O== 4OH£
D£®øƵē³Ų¹¤×÷Ņ»¶ĪŹ±¼äŗóŹÆÄ«°ōµÄÖŹĮæ»į¼õĒį

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠĖÄĘæ¶ŖŹ§±źĒ©µÄ NaOH ”¢Na 2CO3 ”¢AlCl3 ”¢NH4HSO4ČÜŅŗ£¬ĪŖ¼ų±šĖÄĘæČÜŅŗ£¬½«ĖÄĘæČÜŅŗ±ąŗÅĪŖA”¢B”¢C”¢D½ųŠŠŹµŃ锣ŹµŃé¹ż³ĢŗĶ¼ĒĀ¼ČēĶ¼Z4£3ĖłŹ¾(ĪŽ¹ŲĪļÖŹŅŃ¾ĀŌČ„)£ŗ
2CO3 ”¢AlCl3 ”¢NH4HSO4ČÜŅŗ£¬ĪŖ¼ų±šĖÄĘæČÜŅŗ£¬½«ĖÄĘæČÜŅŗ±ąŗÅĪŖA”¢B”¢C”¢D½ųŠŠŹµŃ锣ŹµŃé¹ż³ĢŗĶ¼ĒĀ¼ČēĶ¼Z4£3ĖłŹ¾(ĪŽ¹ŲĪļÖŹŅŃ¾ĀŌČ„)£ŗ
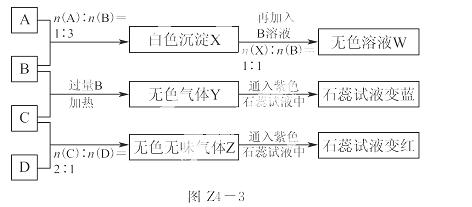
Ēė»Ų“š£ŗ
(1)Y”¢Z µÄ»ÆѧŹ½·Ö±šĪŖ£ŗY__________£»Z__________£»X Óė B ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_________________________”£
(2)DČÜŅŗpH________(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)7£¬ŌŅņŹĒ(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)_____________ _______”£
(3)µČĪļÖŹµÄĮæÅØ¶ČµÄA”¢B”¢C”¢DČÜŅŗpHÓɓ󵽊”µÄĖ³ŠņŹĒ__________(ÓĆ»ÆѧŹ½±ķŹ¾)”£
(4)ĒėŠ“³öCÓė¹żĮæB·“Ó¦(¼ÓČČ)µÄĄė×Ó·½³ĢŹ½£ŗ____________________”£
(5)ČōB”¢CµÄĻ”ČÜŅŗ»ģŗĻŗó(²»¼ÓČČ)ČÜŅŗ³ŹÖŠŠŌ£¬ŌņøĆČÜŅŗÖŠĄė×ÓÅØ¶Č“Ó“óµ½Š”µÄĖ³ŠņŹĒ______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠ¶ĢÖÜĘŚŌŖĖŲ¹¹³ÉµÄ3ÖÖµ„ÖŹ¼×”¢ŅŅ”¢±ū£¬ĖüĆĒŌŚŅ»¶ØĢõ¼žĻĀÄÜ·¢ÉśČēĻĀ±ä»Æ£¬²æ·Ö²śĪļŅŃĀŌČ„”£
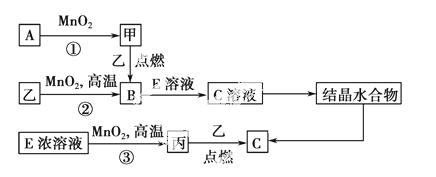
(1)Čō·“Ó¦¢ŁæÉŌŚ³£ĪĀĻĀ½ųŠŠ£¬ŌņAµÄµē×ÓŹ½ĪŖ________”£Čō·“Ó¦¢ŁŠčŌŚ¼ÓČČĢõ¼žĻĀ½ųŠŠ£¬Ōņ·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ĪŖ____________________________”£
(2)·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ________£¬øĆ·“Ó¦ŹĒ________(Ģī”°·ÅČČ”±»ņ”°ĪüČČ”±)·“Ó¦”£
(3)“ÓCČÜŅŗÖŠµĆµ½CŠč¾¹żĮ½²½²Ł×÷£ŗĻČ“ÓCČÜŅŗÖŠµĆµ½¾§Ģ壬ŌŁ½«µĆµ½µÄ¾§Ģå×Ŗ»ÆĪŖC”£“ÓCČÜŅŗÖŠµĆµ½¾§ĢåµÄ¹ż³Ģ±»³ĘĪŖ________£¬½«µĆµ½µÄ¾§Ģå×Ŗ»ÆĪŖCµÄŹµŃéĢõ¼žŹĒ____________________________________”£
(4)·“Ó¦¢ŪÖŠŃõ»Æ¼ĮÓė»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĪļÖŹÖŠ¶¼ŹĒ¼ČÓŠĄė×Ó¼üÓÖÓŠ¹²¼Ū¼üµÄŅ»×éŹĒ(””””)
A£®NaOH”¢H2O”¢NH4Cl
B£®KOH”¢Na2O2”¢(NH4)2S
C£®MgO”¢CaBr2”¢NaCl
D£®Na2SO4”¢HCl”¢MgCl2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖXŌŖĖŲŌ×ÓµÄK”¢L²ćµÄµē×ÓŹżÖ®ŗĶ±ČL”¢M²ćµÄµē×ÓŹżÖ®ŗĶ¶ą1øöµē×Ó”£YŌŖ
ĖŲµÄŌ×Ó×īĶā²ćµē×ÓŹż±ČÄŚ²ćµē×ÓŹżÉŁ3øö”£ZŌŖĖŲŗĖĶāÓŠ3øöµē×Ó²ć£¬×īĶā²ćÓŠ3øöµē×Ó”£WŌŖĖŲ×īøß»ÆŗĻ¼ŪŹĒ×īµĶ»ÆŗĻ¼Ū¾ų¶ŌÖµµÄ3±¶£¬ĖüŌŚ×īøß¼ŪŃõ»ÆĪļÖŠµÄÖŹĮæ·ÖŹżĪŖ40%”£
(1)YŗĶWµÄĘųĢ¬Ēā»ÆĪļµÄĪČ¶ØŠŌĪŖ(ÓĆ»ÆѧŹ½±ķŹ¾)
________£¾________”£
(2)Xµ„ÖŹŌŚæÕĘųÖŠ¼ÓČČÉś³ÉµÄ»ÆŗĻĪļŹĒ________»ÆŗĻĪļ(Ģī”°Ąė×Ó”±»ņ”°¹²¼Ū”±)”£
(3)XŗĶZµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļ·“Ó¦µÄĄė×Ó·½³ĢŹ½_________________________”£
(4)WµÄµĶ¼ŪŃõ»ÆĪļÓėYµ„ÖŹµÄĖ®ČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½________________________”£
(5)YÓėZŠĪ³É»ÆŗĻĪļµÄ»ÆѧŹ½ŹĒ________”£ŹµŃé²āµĆµ±“Ė»ÆŗĻĪļ“¦ÓŚ¹ĢĢ¬ŗĶŅŗĢ¬Ź±²»µ¼µē£¬ČÜÓŚĖ®Äܵ¼µē”£ÓÉ“ĖÅŠ¶ĻøĆ»ÆŗĻĪļ¾ßÓŠ________¼ü(Ģī”°Ąė×Ó”±»ņ”°¹²¼Ū”±)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com