
ij��ѧС����̽�����������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ����ijCuO��Cu��Ϸ�ĩ��CuO����������������ʵ����Ʒ�����Ԥ�ڽ��ۺ������� �� ��
|
| ̽�� | ���� | ���� |
| A | �� | ȡ2mL�����飬��������NaOHˮ��Һ�����ȡ�һ��ʱ�������Һ�еμ�AgNO3��Һ���е���ɫ�������� | �������е�������Br������������NaOHˮ��Һ����ȡ����Ӧ |
| B | �� | ȡ2mL�����飬��������NaOH�Ҵ���Һ�����ȣ�������������ͨ��KMnO4������Һ�У���Һ��ɫ | ��Ӧ������ϩ����������NaOH�Ҵ���Һ������ȥ��Ӧ |
| C | �� | ��ȡ����Ϊm1�Ļ����ڿ����м�ǿ�����������ٱ仯����ȴ�����������ù�������Ϊm2 | CuO����������Ϊ��
|
| D | �� | ��ȡ����Ϊm1�Ļ�����������ϡ�������ܽ⣬���ˡ�ϴ�ӡ����������������������Ϊm2 | CuO����������Ϊ��
|
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
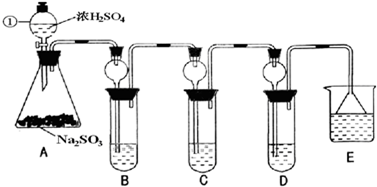
(14��)![]() ��һ�ִ�����Ⱦ�ij��ȤС����̽��
��һ�ִ�����Ⱦ�ij��ȤС����̽��![]() �����ʼ���ɫʵ��ķ�����������·�����
�����ʼ���ɫʵ��ķ�����������·�����
(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
Ϊ��ʵ����ɫʵ���Ŀ�꣬��ͬѧ�������������ͼA����ȡװ�ã�
��Aװ����ȣ�A�� װ�õ��ŵ��ǣ�
�� ��
�� ��
(2)����A�� װ�ú�����������ʵ�顣
I����֤![]() �������ԣ�Cװ���п�ѡ�Լ� (�����)��
�������ԣ�Cװ���п�ѡ�Լ� (�����)��
A��Ba(HCO3)2��Һ B�������� C����ˮ D��Ʒ����Һ
II����֤![]() �Ļ�ԭ�ԣ�Cװ���п����Լ� (������)��
�Ļ�ԭ�ԣ�Cװ���п����Լ� (������)��
�ø��Լ��������Ǣ� ���� ��
(3)Dװ���е����¶˵���©���������� ��
(4)��ҵ���������������Σ��������û������Ʊ�![]() �Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________��
�Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________��
��β���ð����յ�Ŀ���ǣ� ��w^w
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������������2010�������һ��������⣨���ۣ���ѧ���� ���ͣ�ѡ����
ij��ѧС����̽�����������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ����ijCuO��Cu��Ϸ�ĩ��CuO����������������ʵ����Ʒ�����Ԥ�ڽ��ۺ������� �� ��
|
|
̽�� |
���� |
���� |
|
A |
�� |
ȡ2mL�����飬��������NaOHˮ��Һ�����ȡ�һ��ʱ�������Һ�еμ�AgNO3��Һ���е���ɫ�������� |
�������е�������Br������������NaOHˮ��Һ����ȡ����Ӧ |
|
B |
�� |
ȡ2mL�����飬��������NaOH�Ҵ���Һ�����ȣ�������������ͨ��KMnO4������Һ�У���Һ��ɫ |
��Ӧ������ϩ����������NaOH�Ҵ���Һ������ȥ��Ӧ |
|
C |
�� |
��ȡ����Ϊm1�Ļ����ڿ����м�ǿ�����������ٱ仯����ȴ�����������ù�������Ϊm2 |
CuO������������
|
|
D |
�� |
��ȡ����Ϊm1�Ļ�����������ϡ�������ܽ⣬���ˡ�ϴ�ӡ����������������������Ϊm2 |
CuO������������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧС����̽�����������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ����ijCuO��Cu��Ϸ�ĩ��CuO����������������ʵ����Ʒ�����Ԥ�ڽ��ۺ������� �� ��
| ̽�� | ���� | ���� | |
| A | �� | ȡ2mL�����飬��������NaOHˮ��Һ�����ȡ�һ��ʱ�������Һ�еμ�AgNO3��Һ���е���ɫ�������� | �������е�������Br������������NaOHˮ��Һ����ȡ����Ӧ |
| B | �� | ȡ2mL�����飬��������NaOH�Ҵ���Һ�����ȣ�������������ͨ��KMnO4������Һ�У���Һ��ɫ | ��Ӧ������ϩ����������NaOH�Ҵ���Һ������ȥ��Ӧ |
| C | �� | ��ȡ����Ϊm1�Ļ����ڿ����м�ǿ�����������ٱ仯����ȴ�����������ù�������Ϊm2 | CuO����������Ϊ��
|
| D | �� | ��ȡ����Ϊm1�Ļ�����������ϡ�������ܽ⣬���ˡ�ϴ�ӡ����������������������Ϊm2 | CuO����������Ϊ��
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com