
 ����ClCH=CHCl��
����ClCH=CHCl��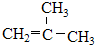 ����
����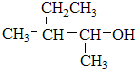 ����
����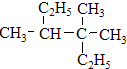 ����
����
 ��
�� ��
�� ���� ��1��2��2��3-���������������4��C��2��2��3�ź��������ݼӾ۷�Ӧ��д���ɣ�
��2����Ϊϩ����������ͬ������ԭ��Ը��л��������������Ϊ����ͬϵ����ݱ���ͬϵ�����������������ɣ�
��3��ͬϵ���ǽṹ���ƣ���������CH2ԭ���ŵ����ʻ���ͬϵ�ͬ���칹���Ƿ���ʽ��ͬ�ṹ��ͬ�����ʣ�ϩ������˳���칹��
��4���ױ������ϵĶ���ȡ������Կ����ױ���2����ԭ�ӱ�2��Hԭ��ȡ�����ױ���Ũ���������¿���Ũ���ᷴӦ����TNT��
��� �⣺��1��2��2��3-���������������4��C��2��2��3�ź������ṹ��ʽΪCH3C��CH3��2CH��CH3��CH3����ClCH=CHCl�Ӿ۷�Ӧ���ɾ�1��2-������ϩ���ṹ��ʽΪ�� ���ʴ�Ϊ��CH3C��CH3��2CH��CH3��CH3��
���ʴ�Ϊ��CH3C��CH3��2CH��CH3��CH3�� ��
��
��2����ѡȡ����̼̼˫�����̼��Ϊ���������л��ﺬ��̼̼˫�����̼������3��C������Ϊ��ϩ��̼̼˫����1��C����2��C����1���������л�������Ϊ��2-��-1-��ϩ����ӱ����ϵ�һ������ʼ��ţ�����ȡ�������֮����С��������λ��Ϊ2��4������Ϊ��1��2��4-���������ʴ�Ϊ��2-��-1-��ϩ��1��2��4-��������
��3����3-��-1-���������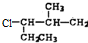 ����ʽ��ͬ���ṹ��ͬ��������ͬ���칹�壻������Ϊ��������������ʽ��ͬ��������ͬϵ��ۺ͢������1���������ҷ���ʽ��ͬ����Ϊ����ͬϵ���Ϊ1��2-������ϩ������˳���칹�壬�ʴ�Ϊ���ڢߣ��٢��ۢ�ݣ�
����ʽ��ͬ���ṹ��ͬ��������ͬ���칹�壻������Ϊ��������������ʽ��ͬ��������ͬϵ��ۺ͢������1���������ҷ���ʽ��ͬ����Ϊ����ͬϵ���Ϊ1��2-������ϩ������˳���칹�壬�ʴ�Ϊ���ڢߣ��٢��ۢ�ݣ�
��4���ױ���3�ֲ�ͬ��Hԭ�ӣ���һ�ȴ�����3�֣��ֱ�Ϊ��λȡ������λȡ���Ͷ�λȡ�����ڴ˻����϶������ȴ���ֱ��У�4�֡�2�֣��ܹ�6�֣��ױ���Ũ���������¿���Ũ���ᷴӦ����TNT����Ӧ�Ļ�ѧ����ʽΪ ���ʴ�Ϊ��6��
���ʴ�Ϊ��6�� ��
��
���� ���⿼�����л��������֪ʶ������ķ����жϣ���Ҫ����������ͬϵ�ͬ���칹���������⣬Ҫ���˽�����������������ͬϵ��������������������������������ʱҪ��ѭ����ԭ����дҪ�淶��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������һ����CH2 ԭ���ŵ��л�����ͬϵ�� | |
| B�� | �����Ԫ������������ͬ������ͬһ���� | |
| C�� | ����ʽ��ͬ���ṹ��ͬ���л��ﲻһ����ͬ���칹�� | |
| D�� | ��Ϊͬϵ����л�������ӽṹ��Ȼ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶��X��Y��Z��W | B�� | �����ԣ�X��Y����ԭ�ԣ�W 2-��Z- | ||
| C�� | ԭ��������������Y��X��Z��W | D�� | ԭ��������Y��X��Z��W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��������Һ������ȩ��ȩ����CH3CHO+2Ag��NH3��2++2OH-$\stackrel{ˮԡ����}{��}$CH3COO-+NH4++3NH3+2Ag��+H2O | |
| B�� | ��CH2BrCOOH�м�������������������Һ�����ȣ�CH2BrCOOH+OH-��$\stackrel{��}{��}$ CH2BrCOO-+H2O | |
| C�� | ϡHNO3ϴ���Թ��е�������3Ag+NO3-+4H+=3Ag++NO��+2H2O | |
| D�� | ������Һ��ͨ��������CO2��CO2+H2O+2C6H5O-��2C6H5OH+2CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ƿ����Һ�ڵζ������н��� | |
| B�� | �ζ���װҺ����첿λ�����ݣ��ζ���������ʧ | |
| C�� | ָʾ����ɫ15s���ָֻ�Ϊԭ������ɫ��ֹͣ�ζ� | |
| D�� | ��ƿ������ˮ��ϴ��δ�ô���Һ��ϴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | E��3s����E��3p����E��3d�� | B�� | E��1s����E��2s����E��3s�� | C�� | E��4f����E��3d����E��4s�� | D�� | E��5s����E��4s����E��4f�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ۼ���ϡ�����У�Fe+2H+=Fe2++H2�� | |
| B�� | Ca��HCO3��2��Һ�����Ca��OH��2��Һ��Ӧ��Ca2++HCO3-+OH-=CaCO3��+H2O | |
| C�� | NH4HCO3��Һ����Ba��OH��2��Һ���ȣ�2HCO3-+2OH-+Ba2+=BaCO3��+CO32-+2H2O | |
| D�� | �ظ������Һ�У�Cr2O72-+H2O?2CrO42-+2H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
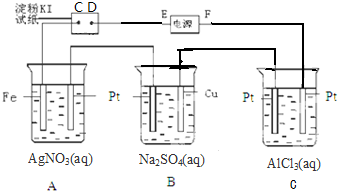
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com