��2012?����һģ��̼���������CH
3OCOOC
2H
5����һ�������﮵���л����Һ������̼���������ԭ��Ϊ��C
2H
5OCOOC
2H
5��g��+CH
3OCOOCH
3��g��?2CH
3OCOOC
2H
5��g����H
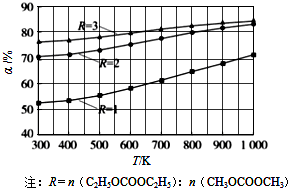
1��1������������ͬ��CH
3OCOOCH
3��ƽ��ת���ʣ��������¶ȣ�T������Ӧ����ȣ�R���Ĺ�ϵ��ͼ��ʾ��
�١�H
1��
��
0�����������=����������
����ͼ��֪��Ϊ�����CH
3OCOOCH
3��ƽ��ת���ʣ��������£���һ��ʩ��
����Ӧ����C2H5OCOOC2H5��Ũ�ȣ��������
����Ӧ����C2H5OCOOC2H5��Ũ�ȣ��������
��
�����ܱ������У���1mol C
2H
5OCOOC
2H
5��1mol CH
3OCOOCH
3��ϼ��ȵ�650K������ͼ�е����ݣ�����¶��¸÷�Ӧ��ƽ�ⳣ��K=
9
9
��
��2����֪������Ӧ��Ҫ���������ڴ��������ͼ�У������д�����������������·�Ӧ��������ϵ�����仯ʾ��ͼ�������б�Ҫ��ע��
��3����֪CH
3OCOOCH
3��g��+C
2H
5OH��g��?CH
3OCOOC
2H
5��g��+CH
3OH��g����H
2CH
3OCOOC
2H
5��g��+C
2H
5OH��g��?C
2H
5OCOOC
2H
5��g��+CH
3OH��g����H
3
��H
1=
��H2��-��H3
��H2��-��H3
���á�H
2�͡�H
3��ʾ��

 ��2012?����һģ��̼���������CH3OCOOC2H5����һ�������﮵���л����Һ������̼���������ԭ��Ϊ��C2H5OCOOC2H5��g��+CH3OCOOCH3��g��?2CH3OCOOC2H5��g����H1
��2012?����һģ��̼���������CH3OCOOC2H5����һ�������﮵���л����Һ������̼���������ԭ��Ϊ��C2H5OCOOC2H5��g��+CH3OCOOCH3��g��?2CH3OCOOC2H5��g����H1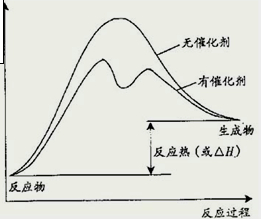 ��
�� ��
��

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�