
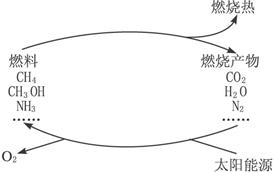
���ֹ�����������·�Ӧ��ʾ��
��2CO2![]() 2CO+O2
2CO+O2
��H2O![]() ___________
___________
��N2+H2O![]() ____________
____________
��CO2+_________CH3OH+_________
��________+H2O![]() CH4_________
CH4_________
(1)�����������ѧ����ʽ��
(2)Ҫʵ��������һЩ���룬Ŀǰ�ͽ��Ҫ����Ĺؼ�������_________��
(3)������ӵ�����������������Ӧ������ɵġ�������Ȼ�Ѿ������������⣬��_________�����ǵ���������Ҫ�Ļ�ѧ��Ӧ֮һ����д����Ӧ�Ļ�ѧ����ʽ��
���������⽫��ѧ֪ʶ�뻷����Ⱦ������������֪ʶ�л���ϵ������˼·Ҫ������Ҫ���롣
���ݹ���ͼ��2CO2![]() 2CO+O2����֪�١��ݾ���O2���ɣ��ٽ�������غ㶨�ɣ�д�����෴Ӧ�Ļ�ѧ����ʽ��Ҳ����д��ȼ��CH4��CH3OH��NH3��H2��O2��Ӧ�ķ���ʽ��������Ӧ��Ҫ����д�ķ���ʽ��
2CO+O2����֪�١��ݾ���O2���ɣ��ٽ�������غ㶨�ɣ�д�����෴Ӧ�Ļ�ѧ����ʽ��Ҳ����д��ȼ��CH4��CH3OH��NH3��H2��O2��Ӧ�ķ���ʽ��������Ӧ��Ҫ����д�ķ���ʽ��
�𰸣�(1)��2H2O![]() 2H2+O2
2H2+O2
��2N2+6H2O![]() 4NH3+3O2
4NH3+3O2
��2CO2+4H2O![]() 2CH3OH+3O2
2CH3OH+3O2
��CO2+2H2O![]() CH4+2CO2
CH4+2CO2
(2)���ʹ�������չ���ת��Ϊ��������
(3)��ɫֲ��Ĺ�����ã������չ������£����ù��ܰѶ�����̼��ˮת���̼ˮ������(����)
6CO2+6H2O![]() C6H12O6+6O2
C6H12O6+6O2


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
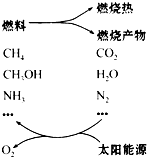 ���������ѳ�Ϊ��ǰ��δ����һ��ȫ�����ش����֮һ��Ϊ����Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���� �ٽ�ȼ��ѭ��ʹ�õĹ��루��ͼ����
���������ѳ�Ϊ��ǰ��δ����һ��ȫ�����ش����֮һ��Ϊ����Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���� �ٽ�ȼ��ѭ��ʹ�õĹ��루��ͼ����| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
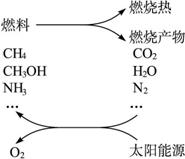
���ֹ�����������·�Ӧ��ʾ��
ͼ4-7
��2CO2![]() 2CO+O2
2CO+O2
��H2O��______________________
��N2+H2O��____________________
��CO2+__________________��CH3OH+_____________________
��______________+H2O��CH4+________________________
��1�������������ѧ����ʽ��
��2��Ҫʵ��������һЩ���룬Ŀǰ�ͽ��Ҫ����Ĺؼ�������_________________________��
��3��������ӵ�����������������Ӧ������ɵġ�������Ȼ�Ѿ������������⣬��_______________________�����ǵ���������Ҫ�Ļ�ѧ��Ӧ֮һ����д����Ӧ�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ѳ�Ϊ��ǰ��δ����һ��ȫ�����ش����֮һ��Ϊ����Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���ܴٽ�ȼ��ѭ��ʹ�õĹ���(��ͼ)��
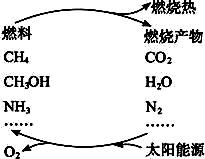
���ֹ�����������·�Ӧ��ʾ��
��2CO2![]() 2CO+O2
2CO+O2
��H2O![]() ____________
____________
��N2+H2O![]() ______________
______________
��CO2+______________![]() CH3OH+_______________
CH3OH+_______________
��__________+H2O![]() CH4+_______________
CH4+_______________
(1)�����������ѧ����ʽ��
(2)Ҫʵ��������һЩ���룬Ŀǰ�ͽ��Ҫ����Ĺؼ�������___________________________��
(3)������ӵ�����������������Ӧ������ɵġ�������Ȼ�Ѿ������������⣬��________ ____________________________________________________________________�����ǵ���������Ҫ�Ļ�ѧ��Ӧ֮һ����д����Ӧ�Ļ�ѧ����ʽ��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ѳ�Ϊ��ǰ��δ����һ��ȫ�����ش����֮һ��Ϊ����Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���ṩ����̫���ܴٽ�ȼ��ѭ��ʹ�õĹ��롣
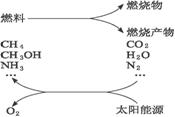
���ֹ�����������·�Ӧ��ʾ��
��2CO2![]() 2CO+O2
2CO+O2
��H2O![]() _______________
_______________
��N2+H2O![]() _______________
_______________
��CO2+_______________![]() CH3OH+_______________
CH3OH+_______________
��_______________+H2O![]() CH4+_______________
CH4+_______________
��1�������������ѧ����ʽ��
��2��Ҫʵ��������һЩ���룬Ŀǰ�ͽ��Ҫ����Ĺؼ�������_________________________
______________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com