
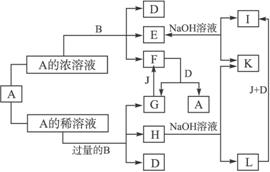
��֪E��Һ��KSCN��ϡ��Һ���ʱ����Һ��Ѫ��ɫ����������и��⣺
��1��B��A��Ũ��Һ��Ӧ��������_____________�����������ѷ�Ӧ��ԭ����____________��
��2��д��F��G�ת���Ļ�ѧ����ʽ��F��G:________________��G��F:________________��
(3)Lת����I��������________________���йصĻ�ѧ����ʽ��________________________��
��1�����ȳ����£�Fe��ŨHNO3�жۻ�
��2��3NO2+H2O====2HNO3+NO
2NO+O2====2NO2
(3)��ɫ������ɻ���ɫ�����ձ�ɺ��ɫ����
4Fe��OH��2+2H2O+O2====4Fe��OH��3
����������ͻ�ƿ���E��Һ��KSCN��ϡ��Һ���ʱ����Һ��Ѫ��ɫ���Ӷ��Ƴ�EΪ���Σ���BΪ����AΪ�ᡣ����ΪFe��A��Ũ��ϡ��Һ��Ӧ���ɲ�ͬ�����ʣ�����A��Ũ��Һ�������ѷ�Ӧ��˵��������ʹFe�ۻ�������AΪHNO3��H2SO4��������Fe��A��Ũ��ϡ��Һ��Ӧ�������������ʣ����й�ͬ������D��˵��AΪHNO3����H2SO4����DΪH2O������A�������B��Ӧ������HΪFe��NO3��2��EΪFe��NO3��3��FΪNO2��GΪNO��JΪO2��KΪNaNO3��IΪFe��OH��3��LΪFe��OH��2��


 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����л�ѧϰ��1 ���ͣ�022
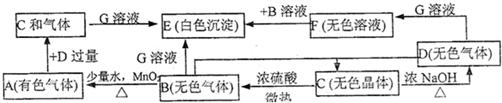
A��B��C��D��E��F��G����ѧ��ѧ�ﳣ������һЩ���ʣ�����֮��������µ�ת����ϵ��

��ͨ���Ըÿ�ͼ�и����ʵ�ת����ϵ�ķ������ش����¸��ʣ�
(1)��ɫ����A�Ļ�ѧʽ��__________����ɫ����C�Ļ�ѧʽ��____________����ɫ����E�Ļ�ѧʽ��______________________��
(2)��Ӧ�ٵĻ�ѧ����ʽ��___________________________��
(3)��Ӧ�ڵĻ�ѧ����ʽ��___________________________��
(4)��Ӧ�۵����ӷ���ʽ��_____________��____________��
(5)��Ӧ�ܵ����ӷ���ʽ��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008������ѧ��һ���ܸ�ϰ֮�ġ�±�ء� ���ͣ�022
A��B��C��D��E��F��G����ѧ��ѧ�ﳣ������һЩ���ʣ�����֮������ͼ��ʾ��ת����ϵ����Щ��Ҫ����Ϣ���ڿ���ע����
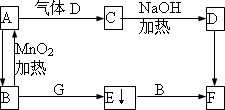
(1)д����ѧʽ��A________��C________��E________��
(2)д����ѧ����ʽ��
A��D��C��________��
B��A��________��
(3)д�����ӷ���ʽ��
F��B��E��________��
B��G��E��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����10�֣�A��B��C��D��E��F��G����ѧ�ﳣ������һЩ���ʣ�����֮��������ת����ϵ����Щ��Ҫ����Ϣ���ڿ�ͼ��ע����
�ش��������⣺
��1��D�Ŀռ乹��Ϊ ��C�ĵ���ʽΪ ��F��Һ������ ��
��2����ʵ��������ȡ����A�����ӷ���ʽ�� ��
��3��д��A��D��C�Ļ�ѧ����ʽ ������������
�������뻹ԭ�����ʵ���֮��Ϊ ��
��4��д����F��B(����)��E�����ӷ���ʽ ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������差��У������ѧ������������ѧ�� ���ͣ������
����10�֣�A��B��C��D��E��F��G����ѧ�ﳣ������һЩ���ʣ�����֮��������ת����ϵ����Щ��Ҫ����Ϣ���ڿ�ͼ��ע����

�ش��������⣺
��1��D�Ŀռ乹��Ϊ ��C�ĵ���ʽΪ ��F��Һ������ ��
��2����ʵ��������ȡ����A�����ӷ���ʽ�� ��
��3��д��A��D��C�Ļ�ѧ����ʽ ������������
�������뻹ԭ�����ʵ���֮��Ϊ ��
��4��д����F��B(����)��E�����ӷ���ʽ ������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com