
��֪�DNH3���ڱ������Լ���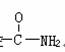 ���ڱ����������ԣ���ѧʽΪC7H7NO2���л���������ӽṹ����һ��������������������λ�����������������Ľṹ��ʽΪ��
���ڱ����������ԣ���ѧʽΪC7H7NO2���л���������ӽṹ����һ��������������������λ�����������������Ľṹ��ʽΪ��
��1��������������� ��
��2��ֻ������ ��
��3��ֻ�м��� ��
��4�������� ��
��5�����ڨDCOOH�ܸ��DNH2�γ� ![]() �����Կ����Ҷ������Ա�����������������������Լ��Ե����ʷ�����Ӧ�����ɸ߷��ӻ�����[(C17H13NO5)]���û�����Ϊ��ǿ����ά���ϣ���ṹ��ʽΪ ��
�����Կ����Ҷ������Ա�����������������������Լ��Ե����ʷ�����Ӧ�����ɸ߷��ӻ�����[(C17H13NO5)]���û�����Ϊ��ǿ����ά���ϣ���ṹ��ʽΪ ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 +2Ag��NH3��2OH
+2Ag��NH3��2OH| �� |
 +2Ag+3NH3+H2O
+2Ag+3NH3+H2O +2Ag��NH3��2OH
+2Ag��NH3��2OH| �� |
 +2Ag+3NH3+H2O
+2Ag+3NH3+H2O

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�






| ||
| ���� |

| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2008��2009ѧ��߶���ѧ��5���¿���ѧ����(A��) ���ͣ�022
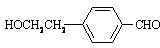
��֪��R��NH2��R1��NH��R2��������NH3���ƣ������ᷴӦ�������ӻ�����л���A�ṹ��ʽ����ͼ��ʾ��

�л���A��ϡ���������Ȼ�ˮ���B��C���ֲ��B��һ�����ӻ����C��һ�ֹ��ۻ����
(1)A�ķ���ʽΪ________����A�Ľṹ��ʽ�����߿��ڵĽṹ����Ϊ________��
(2)д��C��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ________��
(3)C��ͬ���칹���У����ڷ���Ҳ��������Ļ�������________�֣�
(4)ij���л������C��ͬ���칹�壬�������б����ұ�������������ȡ������д�������л����к��������ŵ�����________��
(5)д��B����������������Һ���ȵķ�Ӧ�����ӷ���ʽ________��
�鿴�𰸺ͽ���>>
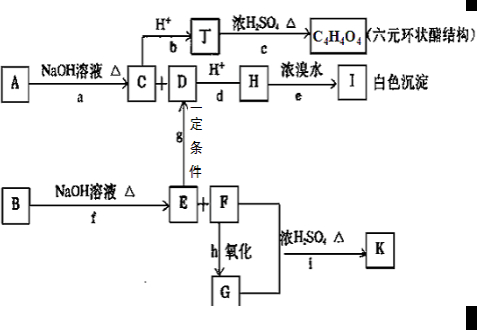
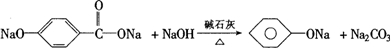
��Ŀ�����л�ѧ ��Դ���Ϻ���ѧ2007������꼶ģ���ԡ���ѧ�Ծ� ���ͣ�022
| |||||||||||||||||||||||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com