

���� A��ʯ���ѽ���Ҫ����֮һ�������������������һ�����ҵ�ʯ�ͻ���ˮƽ��AΪC2H4��B�ܷ�����������������D����BΪ����CΪȩ��DΪ���ᡢEΪ��������ϩ��ˮ�����ӳɷ�Ӧ����B��BΪCH3CH2OH���Ҵ���Cu��Ag�����������·���������ӦCH3CHO��CΪCH3CHO��CH3CHO�ɽ�һ��������CH3COOH��DΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ����������������EΪCH3COOCH2CH3���ݴ˽��
��� �⣺A��ʯ���ѽ���Ҫ����֮һ�������������������һ�����ҵ�ʯ�ͻ���ˮƽ��AΪC2H4��B�ܷ�����������������D����BΪ����CΪȩ��DΪ���ᡢEΪ��������ϩ��ˮ�����ӳɷ�Ӧ����B��BΪCH3CH2OH���Ҵ���Cu��Ag�����������·���������ӦCH3CHO��CΪCH3CHO��CH3CHO�ɽ�һ��������CH3COOH��DΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ����������������EΪCH3COOCH2CH3��
��1��������������֪��A�Ľṹ��ʽΪCH2=CH2��������Ϊˮ���Ĵ������
�ʴ�Ϊ��CH2=CH2���������
��2����ӦB+D��E���Ҵ���������Ũ���ᡢ����������������������������������Ӧ��
�ʴ�Ϊ��������Ӧ��
��3��B��C�ķ�Ӧ����ʽΪ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
B+D��E��Ӧ����ʽΪ��CH3CH2OH+CH3COOH $?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
�ʴ�Ϊ��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O��CH3CH2OH+CH3COOH $?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��4����ȥ�����������������������������ʣ��������Ϊ����E�����Һ©���У��ټ��뱥��̼������Һ����������á��ֲ㣬Ȼ���Һ��ȡ�ϲ�Һ�弴�ɣ�
�ʴ�Ϊ������̼������Һ��
���� ���⿼���л����ƶϡ����ʷ����ᴿ��ϩ�봼��ȩ������֮���ת����ϵ�ȣ��ѶȲ���ע�����֪ʶ���������գ�


 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol/L pH=9��NaNO2��Һ��C��Na+����C��NO2-����C��OH-����C��H+�� | |
| B�� | 0.1mol/LNa2S��Һ�У�2C��Na+��=C��S2-��+C��HS-��+C��H2S�� | |
| C�� | ��PH�İ�ˮ��NaOH��Һ��Ba��OH��2��Һ�У�C��NH4+��=C��Na+��=C��Ba2+�� | |
| D�� | ��NH4HCO3��Һ�еμ�NaOH��Һ��pH=7��C��NH4+��+C��Na+��=C��HCO32-��+C��CO32-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʯ�����Ŀ����ʹԭ�ϳ�����ã�������Ӵ���ʹ��Ӧ���ʼӿ� | |
| B�� | �Ӵ����в��ó�ѹ����Ҫԭ���dz�ѹ��SO2��ת�����Ѿ��ܸ� | |
| C�� | ����¯�г����Ļ������Ҫϴ�ӣ�Ŀ���Ƿ�ֹ�����ж� | |
| D�� | �Ӵ��Ҳ���450����¶���ʹ����������ѣ����ƽ��������SO3�ĺ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ԫ�� | �й����ʻ�ṹ��Ϣ |
| A | �����۵�AԪ�ص��⻯����ͨ��״������һ��Һ�壬����A����������Ϊ88.9% |
| B | Bԭ�ӵõ�һ�����Ӻ�3p���ȫ���� |
| C | Cԭ�ӵ�p����������������̬�⻯������������������ˮ���ﷴӦ����һ�ֳ�������X |
| D | DԪ�ص�����ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣬�����������ˮ���������Ϊͬ������ǿ |
| E | EԪ�صĺ˵��������Aԭ�ӵĺ˵������BԪ���⻯��ĺ˵����֮�� |
 ��д����������������⻯���ˮ��Һ��Ӧ�����ӷ���ʽ��2H2O++H2S=S��+2H2O+2H+��
��д����������������⻯���ˮ��Һ��Ӧ�����ӷ���ʽ��2H2O++H2S=S��+2H2O+2H+���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | ʵ�� | ʵ������ | ���� |
| ʵ��� | ��ʵ��I���ռ����������ȼ | �ܰ���ȼ�ա���������ɫ���� | ����ɷ�Ϊ ���� |
| ʵ��� | ȡʵ��I�еİ�ɫ�����ϴ�ӣ� ��������ϡ���� | �������ݳ���ȫ���ܽ� | ��ɫ��������ܺ���MgCO3 |
| ʵ��� | ȡʵ��I�еij���Һ�������м� ������CaCl2ϡ��Һ | ������ɫ���� | ��Һ�д��ڢ� CO32-���� |
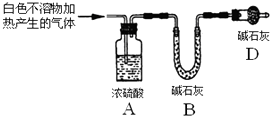
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
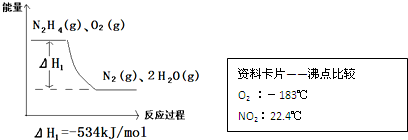
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
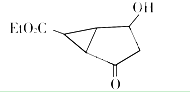
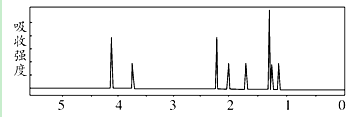
| A�� | ���л��ﲻͬ��ѧ��������ԭ����8�� | |
| B�� | ���л������ڷ����廯���� | |
| C�� | ���л������ʽΪC9H14 O4 | |
| D�� | 1 mol���л�����ȫȼ�տ��Բ���7 molˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��K+��SO42-��MnO4- | B�� | Ca2+��NH4+��Cl-��NO3- | ||
| C�� | Mg2+��K+��HCO3-��Cl- | D�� | Na+��K+��SO32-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com