
 £©£ØF1ŗĶF2»„ĪŖĶ¬·ÖŅģ¹¹Ģ壩£ØG1ŗĶG2»„ĪŖĶ¬·ÖŅģ¹¹Ģ壩
£©£ØF1ŗĶF2»„ĪŖĶ¬·ÖŅģ¹¹Ģ壩£ØG1ŗĶG2»„ĪŖĶ¬·ÖŅģ¹¹Ģ壩 £®
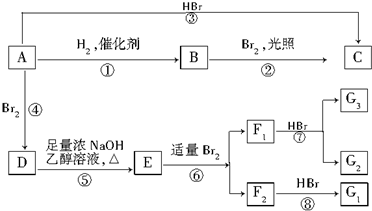
£® ·ÖĪö ÓÉDæÉÖŖAĪŖ£ØCH3£©2C=C£ØCH3£©2£¬ŌņBĪŖ£ØCH3£©2CH-CH£ØCH3£©2£¬ÓÉ·“Ó¦¢ŪæÉÖŖCĪŖ £¬DŌŚNaOH“¼ČÜŅŗÖŠ¼ÓČČĢõ¼žĻĀ·¢ÉśĻūČ„·“Ó¦£¬EĪŖCH2=C£ØCH3£©-C£ØCH3£©=CH2£¬CH2=C£ØCH3£©C£ØCH3£©=CH2æÉÓėäå·¢Éś1£¬2¼Ó³É»ņ1£¬4¼Ó³É£¬F1Óėäå»ÆĒāµĆĮ½ÖÖ²śĪļ£¬¶ųF2Óėäå»ÆĒāµĆŅ»ÖÖ²śĪļ£¬ĒŅF1æÉF2¶¼æÉÉś³ÉG1£¬ŌņF1ĪŖCH2BrCBr£ØCH3£©C£ØCH3£©=CH2£¬F2ĪŖCH2BrC£ØCH3£©=C£ØCH3£©CH2Br£¬G1ĪŖ
£¬DŌŚNaOH“¼ČÜŅŗÖŠ¼ÓČČĢõ¼žĻĀ·¢ÉśĻūČ„·“Ó¦£¬EĪŖCH2=C£ØCH3£©-C£ØCH3£©=CH2£¬CH2=C£ØCH3£©C£ØCH3£©=CH2æÉÓėäå·¢Éś1£¬2¼Ó³É»ņ1£¬4¼Ó³É£¬F1Óėäå»ÆĒāµĆĮ½ÖÖ²śĪļ£¬¶ųF2Óėäå»ÆĒāµĆŅ»ÖÖ²śĪļ£¬ĒŅF1æÉF2¶¼æÉÉś³ÉG1£¬ŌņF1ĪŖCH2BrCBr£ØCH3£©C£ØCH3£©=CH2£¬F2ĪŖCH2BrC£ØCH3£©=C£ØCH3£©CH2Br£¬G1ĪŖ £¬G2ĪŖCH2BrCBr£ØCH3£©CBr£ØCH3£©CH3£¬¾Ż“Ė·ÖĪö½ā“š£®
£¬G2ĪŖCH2BrCBr£ØCH3£©CBr£ØCH3£©CH3£¬¾Ż“Ė·ÖĪö½ā“š£®
½ā“š ½ā£ŗÓÉDæÉÖŖAĪŖ£ØCH3£©2C=C£ØCH3£©2£¬ŌņBĪŖ£ØCH3£©2CH-CH£ØCH3£©2£¬ÓÉ·“Ó¦¢ŪæÉÖŖCĪŖ £¬DŌŚNaOH“¼ČÜŅŗÖŠ¼ÓČČĢõ¼žĻĀ·¢ÉśĻūČ„·“Ó¦£¬EĪŖCH2=C£ØCH3£©-C£ØCH3£©=CH2£¬CH2=C£ØCH3£©C£ØCH3£©=CH2æÉÓėäå·¢Éś1£¬2¼Ó³É»ņ1£¬4¼Ó³É£¬F1Óėäå»ÆĒāµĆĮ½ÖÖ²śĪļ£¬¶ųF2Óėäå»ÆĒāµĆŅ»ÖÖ²śĪļ£¬ĒŅF1æÉF2¶¼æÉÉś³ÉG1£¬ŌņF1ĪŖCH2BrCBr£ØCH3£©C£ØCH3£©=CH2£¬F2ĪŖCH2BrC£ØCH3£©=C£ØCH3£©CH2Br£¬G1ĪŖ
£¬DŌŚNaOH“¼ČÜŅŗÖŠ¼ÓČČĢõ¼žĻĀ·¢ÉśĻūČ„·“Ó¦£¬EĪŖCH2=C£ØCH3£©-C£ØCH3£©=CH2£¬CH2=C£ØCH3£©C£ØCH3£©=CH2æÉÓėäå·¢Éś1£¬2¼Ó³É»ņ1£¬4¼Ó³É£¬F1Óėäå»ÆĒāµĆĮ½ÖÖ²śĪļ£¬¶ųF2Óėäå»ÆĒāµĆŅ»ÖÖ²śĪļ£¬ĒŅF1æÉF2¶¼æÉÉś³ÉG1£¬ŌņF1ĪŖCH2BrCBr£ØCH3£©C£ØCH3£©=CH2£¬F2ĪŖCH2BrC£ØCH3£©=C£ØCH3£©CH2Br£¬G1ĪŖ £¬G2ĪŖCH2BrCBr£ØCH3£©CBr£ØCH3£©CH3£¬
£¬G2ĪŖCH2BrCBr£ØCH3£©CBr£ØCH3£©CH3£¬
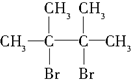
£Ø1£©ĶعżŅŌÉĻ·ÖĪöÖŖ£¬A½į¹¹¼ņŹ½ĪŖ£ØCH3£©2C=C£ØCH3£©2£¬¹Ź“š°øĪŖ£ŗ£ØCH3£©2C=C£ØCH3£©2£»
£Ø2£©·“Ó¦¢ŁĪŖ¼Ó³É·“Ó¦£¬¢ŚĪŖČ”“ś·“Ó¦£¬¢ŪĪŖ¼Ó³É·“Ó¦£¬¢ÜĪŖ¼Ó³É·“Ó¦£¬¢ŻĪŖĻūČ„·“Ó¦£¬¢ŽĪŖ¼Ó³É·“Ó¦£¬¢ßĪŖ¼Ó³É·“Ó¦£¬¢ąĪŖ¼Ó³É·“Ó¦£¬
¹Ź“š°øĪŖ£ŗ¢Ś£»
£Ø3£©æņĶ¼ÖŠ¢Ł”¢¢Ū”¢¢ŽŹōÓŚ¼Ó³É·“Ó¦£¬¹Ź“š°øĪŖ£ŗ¼Ó³É£»
£Ø4£©G1µÄ½į¹¹¼ņŹ½ £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ £®
£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļĶʶĻ£¬ĪŖøßĘµæ¼µć£¬²ąÖŲæ¼²éѧɜ·ÖĪöĶʶĻÄÜĮ¦£¬Ć÷Č·³£¼ūÓŠ»śĪļ¹ŁÄÜĶż°ĘäŠŌÖŹ”¢·“Ó¦Ģõ¼ž”¢·“Ó¦ĄąŠĶŹĒ½ā±¾Ģā¹Ų¼ü£¬ŅŌD½į¹¹¼ņŹ½ĪŖĶ»ĘĘæŚ½ųŠŠĶʶĻ£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Ģ¼ĖįÄĘ£Ø¹ĢĢ壩 | B£® | Ė® | C£® | ĮņĖį¼ŲČÜŅŗ | D£® | ĮņĖįļ§£Ø¹ĢĢ壩 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
 £¬ĻĀĮŠÓŠ¹ŲøĆĪļÖŹŠšŹöÕżČ·µÄŹĒ£Ø””””£©
£¬ĻĀĮŠÓŠ¹ŲøĆĪļÖŹŠšŹöÕżČ·µÄŹĒ£Ø””””£©| A£® | ²»ÄÜÓė½šŹōÄĘ·¢Éś·“Ó¦ | |
| B£® | ²»ÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ« | |
| C£® | ŌŚÅØĮņĖį“ß»ÆĻĀ£®ÄÜÓėŅŅĖį·¢Éś·“Ó¦ | |
| D£® | ³£ĪĀĻĀÄÜÓėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ·¢Éś¼Ó³É·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| ŌŖĖŲ±ąŗÅ ŌŖĖŲŠŌÖŹ | ¢Ł | ¢Ś | ¢Ū | ¢Ü | ¢Ż | ¢Ž | ¢ß | ¢ą |
| Ō×Ó°ė¾¶£Ø10-10m£© | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 1.43 |
| ×īøß»ņ×īµĶ»ÆŗĻ¼Ū | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -3 | -1 | -3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH3CH2CH3 | B£® | CH3-O-CH3 | C£® | 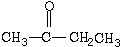 | D£® | 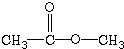 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| Al3+ | Fe3+ | AlO2- | SiO32- | |
| æŖŹ¼³ĮµķŹ± | 3.4 | 1.9 | 10.6 | 7.3 |
| ³ĮµķĶźČ«Ź± | 4.7 | 3.2 | 9.3 | 5.3 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | O2ÓėO3 | B£® | 35ClÓė37Cl | C£® | 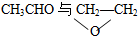 | D£® | CH4ÓėC2H6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
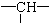 £¬ĘäÓąĪŖĀČŌ×Ó£®ŌņĀČŌ×ÓµÄøöŹżĪŖ£Ø””””£©
£¬ĘäÓąĪŖĀČŌ×Ó£®ŌņĀČŌ×ÓµÄøöŹżĪŖ£Ø””””£©| A£® | 2y+3x-x | B£® | z+2-x | C£® | 2y+z-x | D£® | z+2y+2-x |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com