
������Ҫ���л��ϳ��м��壬��ش��������⣺
��1��ʵ������ȡ����������ʵ���У���ƿ�г������Ҵ���Ũ�������ˮ�����⣬��Ӧ�������Ƭ����Ŀ���� ����ƿ�з����ķ�Ӧ�Ļ�ѧ����ʽΪ ��
��2������������ת���ʣ��ɲ�ȡ�Ĵ�ʩ�� �� �ȡ�
 ��3����ƿ���ռ������������ֲ�Ʒ����������
��3����ƿ���ռ������������ֲ�Ʒ����������
��Ҫ�� ��
��4��ʵ��ʱ�ɹ۲쵽��ƿ�������ݲ�����
�����ӷ���ʽ��ʾ�������ݵ�ԭ�� ��
��5���˷�Ӧ��Ũ������Ϊ���������ܻ���ɲ����������Է�Һ�������ظ�ʹ�����ѵ����⡣�ִ��о���������������Һ������˷�Ӧ�Ĵ�����ʵ���������±���ʾ��������Ҵ��Ե����ʵ�����ϣ���
| ͬһ��Ӧʱ�� | ͬһ��Ӧ�¶� | ||||
| ��Ӧ�¶�/�� | ת����(%) | ѡ����(%) | ��Ӧʱ��/h | ת����(%) | ѡ����(%) |
| 40 | 77.8 | 100 | 2 | 80.2 | 100 |
| 60 | 92.3 | 100 | 3 | 87.7 | 100 |
| 80 | 92.6 | 100 | 4 | 92.3 | 100 |
| 120 | 94.5 | 98.7 | 6 | 93.0 | 100 |
��˵����ѡ����100%��ʾ��Ӧ���ɵIJ���������������ˮ��
���ݱ������ݣ�����_________(����)��Ϊ�÷�Ӧ�����������
A. 120�棬4h B. 80�棬2h C. 60�棬4h D. 40�棬3h

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼ��װ���Ʊ�����������
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼ��װ���Ʊ�����������| ʵ���� | �Թܢ����Լ� | �Թܢ����Լ� | ����л���ĺ��/cm |
| A | 3mL�Ҵ���2mL���ᡢ1mL18mol?L-1 Ũ���� | ����Na2CO3��Һ | 5.0 |
| B | 3mL�Ҵ���2mL���� | 0.1 | |
| C | 3mL�Ҵ���2mL���ᡢ6mL 3mol?L-1 H2SO4 | 1.2 | |
| D | 3mL�Ҵ���2mL���ᡢ���� | 1.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH
������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH | Ũ���� | �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | ������ɫ��̬ | �ܶȣ�g/ml�� | �۵㣨�棩 | �е㣨�棩 |
| ������ | ��ɫ���� | 1.2659 | 122 | 249 |
| �״� | ��ɫҺ�� | 0.7915 | -97.8 | 64.65 |
| ��������� | ��ɫҺ�� | 1.0888 | -12.3 | 199.6 |
| ���� | ��ɫҺ�� | \ | 16.6 | 117.9 |
| �Ҵ� | ��ɫҺ�� | \ | -117.3 | 78.5 |
| �������� | ��ɫҺ�� | \ | 83.6 | 77.1 |

| ŨH2SO4 |
| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����ñ����ᡢ��ˮ�Ҵ���Ũ�����ϣ��ڼ��������·�Ӧ�Ƶã�
������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����ñ����ᡢ��ˮ�Ҵ���Ũ�����ϣ��ڼ��������·�Ӧ�Ƶã�| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
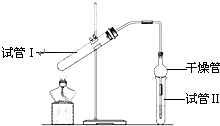 ������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ��������ͼ��װ���Ʊ�����������
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ��������ͼ��װ���Ʊ�����������| ʵ���� | �Թܢ����Լ� | �Թܢ��� �Լ� |
���� ���/cm |
| A | 2mL�Ҵ���1mL���ᡢ 1mL18mol?L-1 Ũ���� |
����Na2CO3 ��Һ |
3.0 |
| B | 2mL�Ҵ���1mL���� | 0.1 | |
| C | 2mL�Ҵ���1mL���ᡢ 3mL 2mol?L-1H2SO4 |
0.6 | |
| D | 2mL�Ҵ���1mL���ᡢ���� | 0.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com