

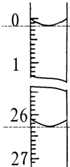
·ÖĪö £Ø1£©øł¾ŻSO3µÄČŪµćŹĒ16.8”ę£¬·ŠµćŹĒ44.8”ęæÉÖŖ±łĖ®Ź¹ČżŃõ»ÆĮņĘųĢå±ä³É¹ĢĢå£»ČżŃõ»ÆĮņÓĆĖ®ĪüŹÕ²śÉśĖįĪķ£»
£Ø2£©BÖŠ³öĻÖ¾§Ģ壬CÖŠ³öĻÖ»ė×Ē£¬ĖµĆ÷ĪŽČżŃõ»ÆĮņ²śÉś£¬Ź¹ŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢåŹĒ¶žŃõ»ÆĮņ£¬ĮņĖįĶÖŠĮņŌŖĖŲ»ÆŗĻ¼Ū½µµĶ£¬Ö»ÄÜŹĒŃõŌŖĖŲ»ÆŗĻ¼ŪÉżøߣ¬²śÉśŃõĘų£»
£Ø3£©ĪŖĮĖÖ¤Ć÷ŹµŃéÓė¶žŃõ»ÆĢ¼ÓŠ¹Ų£¬ŠčŅŖŌŚ³żµō¶žŃõ»ÆĮņµÄĒ°ĢįĻĀ¹Ū²ģŹÆ»ŅĖ®ŹĒ·ń±ä»ė×Ē£»
£Ø4£©ÓƵČÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗµĪ¶ØŃĒĮņĖįČÜŅŗ£¬ĻūŗÄĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żŹĒŃĒĮņĖįµÄ2±¶£®
½ā“š ½ā£ŗ£Ø1£©øł¾ŻSO3µÄČŪµćŹĒ16.8”ę£¬·ŠµćŹĒ44.8”ęæÉÖŖ±łĖ®Ź¹ČżŃõ»ÆĮņĘųĢå±ä³É¹ĢĢå£»ČżŃõ»ÆĮņÓĆĖ®ĪüŹÕ²śÉśĖįĪķ£¬
¹Ź“š°øĪŖ£ŗSO3£»²śÉśĖįĪķ£»
£Ø2£©BÖŠ³öĻÖ¾§Ģ壬CÖŠ³öĻÖ»ė×Ē£¬ĖµĆ÷ĪŽČżŃõ»ÆĮņ²śÉś£¬Ź¹ŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢåŹĒ¶žŃõ»ÆĮņ£¬ĮņĖįĶÖŠĮņŌŖĖŲ»ÆŗĻ¼Ū½µµĶ£¬Ö»ÄÜŹĒŃõŌŖĖŲ»ÆŗĻ¼ŪÉżøߣ¬²śÉśŃõĘų£¬»Æѧ·½³ĢŹ½ĪŖ£ŗ2CuSO4$\frac{\underline{\;\;”÷\;\;}}{\;}$ 2CuO+2SO2”ü+O2”ü£¬
¹Ź“š°øĪŖ£ŗ2CuSO4$\frac{\underline{\;\;”÷\;\;}}{\;}$ 2CuO+2SO2”ü+O2”ü£»
£Ø3£©¢ŁĪŖĮĖÖ¤Ć÷ŹµŃéÓė¶žŃõ»ÆĢ¼ÓŠ¹Ų£¬ŠčŅŖŌŚ³żµō¶žŃõ»ÆĮņµÄĒ°ĢįĻĀ¹Ū²ģŹÆ»ŅĖ®ŹĒ·ń±ä»ė×Ē£¬³ż¶žŃõ»ÆĮņÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£¬aÖŠŹŌ¼ĮĪŖĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£¬
¹Ź“š°øĪŖ£ŗĖįŠŌKMnO4ČÜŅŗ£»
¢ŚaÖŠŹŌ¼ĮĪŖĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£¬³żµō¶žŃõ»ÆĮņ£¬ĪŖČ·±£³ż¾”ÓĆĘ·ŗģĄ“¼ģŃ飬µ±bÖŠĘ·ŗģ²»ĶŹÉ«£¬×°ÖĆcÖŠ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬¼“Ö¤Ć÷ĮĖ¶žŃõ»ÆĢ¼µÄøÉĀ££¬
¹Ź“š°øĪŖ£ŗ¼ģŃ鶞Ńõ»ÆĮņŹĒ·ń±»³ż¾”£»µ±bÖŠĘ·ŗģ²»ĶŹÉ«£¬×°ÖĆcÖŠ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£»
£Ø4£©ÓƵČÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗµĪ¶ØŃĒĮņĖįČÜŅŗ£¬ĻūŗÄĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żŹĒŃĒĮņĖįµÄ2±¶£¬ŌņÖ¤Ć÷ĮĖŃĒĮņĖįŹĒ¶žŌŖĖį£¬
¹Ź“š°øĪŖ£ŗÓƵČÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗµĪ¶ØŃĒĮņĖįČÜŅŗ£¬ĻūŗÄĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żŹĒŃĒĮņĖįµÄ2±¶£¬ŌņÖ¤Ć÷ĮĖŃĒĮņĖįŹĒ¶žŌŖĖį£®
µćĘĄ ±¾ĢāĢ½¾æĮņĖįĶŹÜ·Ö½āµĆµ½²śĪļŹĒŹ²Ć“£¬Ķعż¶ØŠŌŹµŃé²¢½įŗĻŃõ»Æ»¹Ō·“Ó¦µÄ¹ęĀÉĶʶĻ³ö²śĪļ£¬°üĄØŹµŃéÉč¼Ę£¬¶¼æ¼²éĮĖѧɜŌĖÓĆÖŖŹ¶µÄÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĢģČ»µ°°×ÖŹÖŠ½öŗ¬C”¢H”¢0”¢NĖÄÖÖŌŖĖŲ | |
| B£® | ¼ÓČė£ØNH4£©2S04±„ŗĶČÜŅŗ»įŹ¹µ°°×ÖŹ±äŠŌ | |
| C£® | ÖŲ½šŹōŃĪÄÜŹ¹µ°°×ÖŹÄż½į£¬ĖłŅŌĪóŹ³ÖŲ½šŹōŃĪ»įÖŠ¶¾ | |
| D£® | ¼¦µ°Ēå¼ÓČėŹ³ŃĪ£¬»įŹ¹µ°°×ÖŹ±äŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Ä³Ń§ÉśÓūÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖįČ„²ā¶ØŌŚæÕĘųÖŠĀ¶ÖĆŅ»¶ĪŹ±¼äŗóµÄNaOH¹ĢĢåµÄ“æ¶Č£®Éč¼ĘČēĻĀ·½°ø£ŗ
Ä³Ń§ÉśÓūÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖįČ„²ā¶ØŌŚæÕĘųÖŠĀ¶ÖĆŅ»¶ĪŹ±¼äŗóµÄNaOH¹ĢĢåµÄ“æ¶Č£®Éč¼ĘČēĻĀ·½°ø£ŗ| µĪ¶Ø “ĪŹż | “ż²āNaOHČÜŅŗµÄĢå»ż/mL | 0.1000mol/LŃĪĖįµÄĢå»ż/mL | ||
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | Ę½¾łŗÄÓĆŃĪĖį Ģå»ż/mL | ||
| µŚŅ»“Ī | 25.00 | 0.00 | 26.15 | ¢ŁV=26.20 |
| µŚ¶ž“Ī | 25.00 | 0.56 | 30.30 | |
| µŚČż“Ī | 25.00 | 0.20 | 26.45 | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻņijĪļÖŹÖŠµĪ¼ÓŃĪĖįŗó£¬ÓŠÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×ĒµÄĪŽÉ«ĪŽĪ¶ĘųĢå²śÉś£¬Ö¤Ć÷ŗ¬CO32- | |
| B£® | ½«Ä³»ÆŗĻĪļ½ųŠŠŃęÉ«ŹµŃ飬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģ£¬ŃęÉ«ĪŖ×ĻÉ«£¬Ö¤Ć÷ŗ¬¼Ų | |
| C£® | “ż¼ģŅŗÖšµĪ¼ÓČėNaOHČÜŅŗ£¬ÓŠ°×É«½ŗד³Įµķ²śÉś£¬ŗóĄ“³ĮµķÖš½„ĻūŹ§£¬ŌņŌČÜŅŗÖŠæÉÄÜŗ¬ÓŠAl3+ | |
| D£® | “ż¼ģŅŗÖŠĻČ¼ÓČėKSCNČÜŅŗ£¬ĪŽĆ÷ĻŌĻÖĻó£¬ŌŁ¼ÓČėĀČĖ®£¬ČÜŅŗĻŌŃŖŗģÉ«£¬Ōņ“ż¼ģŅŗÖŠŅ»¶Øŗ¬ÓŠFe2+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ņ»¶ØĢõ¼žĻĀ£¬½«1 mol N2ŗĶ3 mol H2»ģŗĻ·¢Éś·“Ó¦£¬×ŖŅʵĵē×Ó×ÜŹżĪŖ6 NA | |
| B£® | 1 L 0.1 mol•L-1µÄNa2CO3ČÜŅŗÖŠŅõĄė×ÓµÄ×ÜŹż“óÓŚ0.1 NA | |
| C£® | ±ź×¼×“æöĻĀ£¬44.8LHFÖŠŗ¬ÓŠ·Ö×ӵďżÄæĪŖ2NA | |
| D£® | 1 mol-CH3ÖŠĖłŗ¬µÄµē×Ó×ÜŹżĪŖ10 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
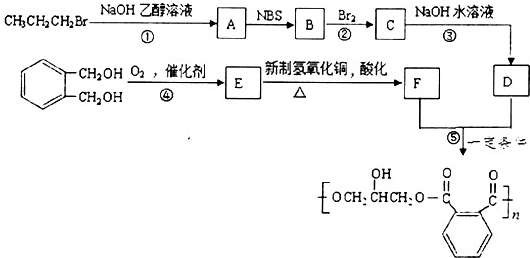
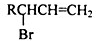
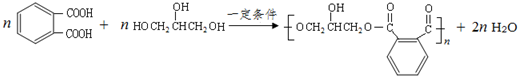
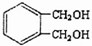 µÄĶ¬·ÖŅģ¹¹ĢåÖŠĶ¬Ź±·ūŗĻĻĀĮŠĢõ¼žµÄ·¼Ļć×å»ÆŗĻĪļ£¬ŹŌŠ“³öĘäÖŠŅ»ÖֵĽį¹¹¼ņŹ½
µÄĶ¬·ÖŅģ¹¹ĢåÖŠĶ¬Ź±·ūŗĻĻĀĮŠĢõ¼žµÄ·¼Ļć×å»ÆŗĻĪļ£¬ŹŌŠ“³öĘäÖŠŅ»ÖֵĽį¹¹¼ņŹ½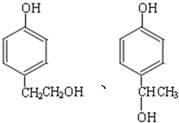 £ØĘäÖŠÖ®Ņ»£©£»
£ØĘäÖŠÖ®Ņ»£©£»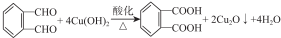 £»
£» £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
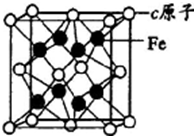 a”¢b”¢c”¢dŹĒĖÄÖÖŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚŌŖĖŲ£®aŌ×ӵĵē×Ó²ćŹżĪŖn£¬ŗĖÄŚÖŹ×ÓŹżŹĒ2n2-1£¬×īĶā²ćµē×ÓŹżĪŖ2n+l£»b”¢dĶ¬Ö÷×壬ÄÜŠĪ³ÉĮ½ÖÖ֊ѧ³£¼ūµÄ»ÆŗĻĪļ£»cÓėb×é³ÉµÄ»ÆŗĻĪļŹĒŅ»ÖÖĮ½ŠŌŃõ»ÆĪļ£¬¹¤ŅµÉĻĶعżµē½āøĆ»ÆŗĻĪļæÉŅ±Į¶cµ„ÖŹ£»eŌ×ÓÓŠĖÄøöÄÜ²ć£¬ĘäĪ“³É¶Ōµē×ÓŹżŌŚĶ¬ÖÜĘŚŹĒ×ī¶ąµÄ£®»Ų“šĻĀĮŠĪŹĢā£ŗ
a”¢b”¢c”¢dŹĒĖÄÖÖŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚŌŖĖŲ£®aŌ×ӵĵē×Ó²ćŹżĪŖn£¬ŗĖÄŚÖŹ×ÓŹżŹĒ2n2-1£¬×īĶā²ćµē×ÓŹżĪŖ2n+l£»b”¢dĶ¬Ö÷×壬ÄÜŠĪ³ÉĮ½ÖÖ֊ѧ³£¼ūµÄ»ÆŗĻĪļ£»cÓėb×é³ÉµÄ»ÆŗĻĪļŹĒŅ»ÖÖĮ½ŠŌŃõ»ÆĪļ£¬¹¤ŅµÉĻĶعżµē½āøĆ»ÆŗĻĪļæÉŅ±Į¶cµ„ÖŹ£»eŌ×ÓÓŠĖÄøöÄÜ²ć£¬ĘäĪ“³É¶Ōµē×ÓŹżŌŚĶ¬ÖÜĘŚŹĒ×ī¶ąµÄ£®»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com