
 ��
�� ��
�� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ��� | �� | �� | �� | �� | �� | �� | �� |
| �� �� �� װ �� |  | 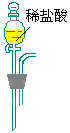 |  | 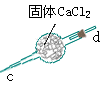 |  |  |  |
 CO3��Ʒ�е�NaHCO3�ֽ�ų��Ķ�����̼�������
CO3��Ʒ�е�NaHCO3�ֽ�ų��Ķ�����̼��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
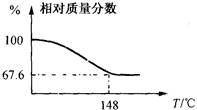
 ����Na2CO4�������ϴ����������Ŀ���Ƕ��������Ư�ס����������û�ѧ����ʽ��ʾ����ϴ��ԭ���� ��
����Na2CO4�������ϴ����������Ŀ���Ƕ��������Ư�ס����������û�ѧ����ʽ��ʾ����ϴ��ԭ���� ��| A��MnO2 | B��H2S | C��CH3COOH | D��NaHCO3 |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | 50 mL���� | 50 mL���� | 50 mL���� |
| m(�����) | 9.2 g | 15.7 g | 27.6 g |
| V(CO2)(��״��) | 2.24 L | 3.36 L | 3.36 L |
| A����������ʵ���Ũ��Ϊ3.0 mol��L��1 | B�����ݱ��������ܼ�����������NaHCO3���������� |
| C����������9.2 gʱ������� | D��15.7 g�����ǡ����������ȫ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1�U4 | B��1�U5 | C��2�U1 | D��2�U3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A������Һ�����ʵ���Ũ�ȣ�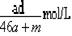 |
B�������������һ��Ϊ44. 8a L 8a L |
C������Һ������������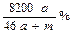 |
| D������Һ��ͨ�����CO2��δ����ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
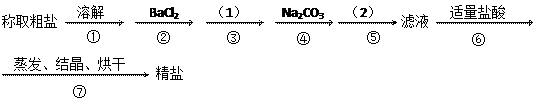
 �ںܿ͢ɷ�ߵ�_
�ںܿ͢ɷ�ߵ�_ ___________����ǡ������������˵�����ɡ�___________________________________________��
___________����ǡ������������˵�����ɡ�___________________________________________�� �����ݣ�����ʵ��
�����ݣ�����ʵ�� �������Ӱ�죬��ԭ���ǣ�
�������Ӱ�죬��ԭ���ǣ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com