
ijļ§Ģ¬µŖ·ŹÓÉW”¢X”¢Y”¢Z 4ÖÖ¶ĢÖÜĘŚŌŖĖŲ×é³É£¬ĘäÖŠWµÄŌ×Ó°ė¾¶×īŠ””£
¢ń.ČōY”¢ZĶ¬Ö÷×壬ZY2ŹĒŠĪ³ÉĖįÓźµÄÖ÷ŅŖĪļÖŹÖ®Ņ»”£
£Ø1£©½«X”¢Y”¢ZµÄŌŖĖŲ·ūŗÅĢīŌŚČēĶ¼ĖłŹ¾ŌŖĖŲÖÜĘŚ±ķ£Ø¾Ö²æ£©ÖŠµÄĻąÓ¦Ī»ÖĆÉĻ”£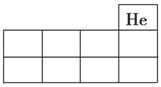
£Ø2£©XµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĻ”ČÜŅŗÓėĶ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©Ņ»¶ØĢõ¼žĻĀ£¬1 mol XW3ĘųĢåÓėO2ĶźČ«·“Ӧɜ³ÉXŌŖĖŲµÄµ„ÖŹŗĶŅŗĢ¬Ė®£¬·Å³ö382.8 kJČČĮ攣øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ __”£
¢ņ.ČōZŹĒŠĪ³É»ÆŗĻĪļÖÖĄą×ī¶ąµÄŌŖĖŲ”£
£Ø4£©øƵŖ·ŹµÄĆū³ĘŹĒ __£ØĢīŅ»ÖÖ£©”£
£Ø5£©HRŹĒŗ¬ZŌŖĖŲµÄŅ»ŌŖĖį”£ŹŅĪĀŹ±£¬ÓĆ0.250 mol”¤L£1NaOHČÜŅŗµĪ¶Ø25.0 mL HRČÜŅŗŹ±£¬ČÜŅŗµÄpH±ä»ÆĒéæöČēĶ¼ĖłŹ¾”£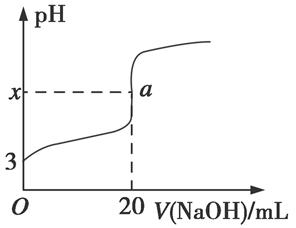
ĘäÖŠ£¬aµć±ķŹ¾Į½ÖÖĪļÖŹĒ”ŗĆĶźČ«·“Ó¦”£
¢ŁĶ¼ÖŠx £ØĢī”°£¾”±”°£¼”±»ņ”°£½”±£©7”£
¢ŚŹŅĪĀŹ±£¬HRµÄµēĄė³£ŹżKa£½ £ØĢīŹżÖµ£©”£
 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
A”¢B”¢C”¢DŹĒ³£¼ūµÄ¶ĢÖÜĘŚŌŖĖŲ£¬øł¾ŻÓŠ¹ŲŠÅĻ¢£¬°“ŅŖĒó»Ų“šĪŹĢā£ŗ
ŠÅĻ¢¢Ł£ŗA”¢B”¢C”¢DŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬ĒŅŌ×ÓŗĖĶā×īĶā²ćµē×ÓŹż¾ł²»ÉŁÓŚ2£»
£Ø1£©øł¾ŻŠÅĻ¢¢Ł£ŗAŅ»¶Ø²»ŹĒ £ØĢīŠņŗÅ£©£»
A£®Ēā B£® Ģ¼ C£®Ńõ D£®Įņ
ŠÅĻ¢¢Ś£ŗÉĻŹöĖÄÖÖŌŖĖŲµÄµ„ÖŹ³£ĪĀĻĀ¾łĪŖ¹ĢĢ壬Ęä×īøß¼ŪŃõ»ÆĪļÖŠÓŠĮ½ÖÖÄÜČÜÓŚĻ”ĮņĖį£¬ČżÖÖÄÜČÜÓŚÅØĒāŃõ»ÆÄĘČÜŅŗ£»
£Ø2£©ÕāĖÄÖÖŌŖĖŲÖŠŹĒ·ńæÉÄÜÓŠŅ»ÖÖŹĒĀĮŌŖĖŲ £ØĢī”°æÉÄÜ”±»ņ”°²»æÉÄÜ”±£©£»
ŠÅĻ¢¢Ū£ŗAÓėDĶ¬Ö÷×壬¹¤ŅµÉĻ³£ÓĆAøßĪĀĻĀ»¹ŌDO2ÖĘČ”D£»
£Ø3£©AÓėDO2·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»
ŠÅĻ¢¢Ü£ŗĻņÉĻŹöĖÄÖÖŌŖĖŲµÄµ„ÖŹ×é³ÉµÄ»ģŗĻĪļÖŠ¼ÓČė×ćĮæŃĪĖįČÜŅŗ£¬¹ĢĢå²æ·ÖČܽā£¬¹żĀĖŗóĻņĀĖŅŗÖŠ¼ÓČė¹żĮæµÄÉÕ¼īČÜŅŗ£¬×īÖÕČÜŅŗÖŠĪö³ö°×É«³Įµķ£»
£Ø4£©°×É«³ĮµķĪļµÄ»ÆѧŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
I£®¶ĢÖÜĘŚŌŖĖŲX”¢Y”¢Z”¢WŌŚŌŖĖŲÖÜĘŚ±ķÖŠĻą¶ŌĪ»ÖĆČēĶ¼ĖłŹ¾£¬ĘäÖŠYĖł“¦µÄÖÜĘŚŠņŹżÓė×åŠņŹżĻąµČ”£°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öXµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼_______________”£
£Ø2£©ĮŠ¾ŁŅ»øöŹĀŹµĖµĆ÷W·Ē½šŹōŠŌĒæÓŚZ: _______________(ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾)”£
£Ø3£©ŗ¬YµÄijÖÖŃĪ³£ÓĆ×÷¾»Ė®¼Į£¬Ęä¾»Ė®ŌĄķŹĒ__________(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)”£
II£®ŌĖÓĆĖłŃ§»ÆѧŌĄķ£¬½ā¾öĻĀĮŠĪŹĢā£ŗ
£Ø4£©ŅŃÖŖ£ŗSi+2NaOH+H2O£½Na2SiO3+2H2”£Ä³Ķ¬Ń§ĄūÓƵ„ÖŹ¹čŗĶĢśĪŖµē¼«²ÄĮĻÉč¼ĘŌµē³Ų(NaOHĪŖµē½āÖŹČÜŅŗ)£¬øĆŌµē³Ųøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ_________________”£
£Ø5£©ŅŃÖŖ£ŗ¢ŁC(s)+ O2(g)£½CO2(g) 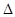 H£½a kJ”¤ mol-1£»¢ŚCO2(g) +C(s)£½2CO(g)
H£½a kJ”¤ mol-1£»¢ŚCO2(g) +C(s)£½2CO(g) 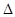 H£½b kJ”¤ mol-1£»¢ŪSi(s)+ O2(g)£½SiO2(s)
H£½b kJ”¤ mol-1£»¢ŪSi(s)+ O2(g)£½SiO2(s) 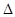 H£½c kJ”¤ mol-1”£¹¤ŅµÉĻÉś²ś“Ö¹čµÄČČ»Æѧ·½³ĢŹ½ĪŖ____________”£
H£½c kJ”¤ mol-1”£¹¤ŅµÉĻÉś²ś“Ö¹čµÄČČ»Æѧ·½³ĢŹ½ĪŖ____________”£
£Ø6£©ŅŃÖŖ£ŗCO(g)+H2O(g) H2(g) + CO2(g)”£ÓŅ±ķĪŖøĆ·“Ó¦ŌŚ²»Ķ¬ĪĀ¶ČŹ±µÄĘ½ŗā³£Źż”£Ōņ£ŗøĆ·“Ó¦µÄ
H2(g) + CO2(g)”£ÓŅ±ķĪŖøĆ·“Ó¦ŌŚ²»Ķ¬ĪĀ¶ČŹ±µÄĘ½ŗā³£Źż”£Ōņ£ŗøĆ·“Ó¦µÄ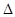 H________0(Ģī”°£¼”±»ņ”°£¾”±)£»500”ꏱ½ųŠŠøĆ·“Ó¦£¬ĒŅCOŗĶH2OĘšŹ¼ÅضČĻąµČ£¬COĘ½ŗā×Ŗ»ÆĀŹĪŖ_________”£
H________0(Ģī”°£¼”±»ņ”°£¾”±)£»500”ꏱ½ųŠŠøĆ·“Ó¦£¬ĒŅCOŗĶH2OĘšŹ¼ÅضČĻąµČ£¬COĘ½ŗā×Ŗ»ÆĀŹĪŖ_________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚ1961Äź£¬±¾ÉśŗĶ»ł¶ū»ō·ņ·¢ĻÖĮĖŅ»ÖÖŠĀµÄ¼ī½šŹōŌŖĖŲ£¬øł¾ŻŅŃѧÖŖµĄ½ā“šĻĀĮŠĪŹĢā£¬°ļÖśÕāĮ½Ī»æĘѧ¼ŅŃŠ¾æøĆŌŖĖŲ”£
£Ø1£©øĆŌŖĖŲµ„ÖŹµÄĆܶȱČĖ®µÄĆܶȓó£¬ŌņøĆŌŖĖŲæÉÄÜŹĒ_______________________________”£
£Ø2£©øĆŌŖĖŲµÄµ„ÖŹÓėĖ®·“Ó¦±Č¼ŲÓėĖ®·“Ó¦¾ēĮŅ£¬µ«Ć»ÓŠļ¤ÓėĖ®·“Ó¦¾ēĮŅ£¬ÓÉ“ĖæÉČ·¶ØøĆŌŖĖŲŹĒ________”£ĘäČ·¶ØŅĄ¾ŻŹĒ_____________________________________________________”£
£Ø3£©ČĖĄąÖʱø³öøĆŌŖĖŲµÄµ„ÖŹ±ČÄʵ„ÖŹŅŖĶķµĆ¶ą£¬·ÖĪöĘäÖŠµÄŌŅņ£ŗ__________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FĮłÖÖ¶ĢÖÜĘŚŌŖĖŲÖŠ£¬A”¢B”¢C”¢DŹĒ×é³Éµ°°×ÖŹµÄ»ł±¾ŌŖĖŲ£»AÓėBµÄŌ×ÓŠņŹżÖ®ŗĶµČÓŚCŌ×ÓŗĖÄŚµÄÖŹ×ÓŹż£»AÓėE”¢DÓėF·Ö±šĪ»ÓŚĶ¬Ņ»Ö÷×壬ĒŅFŌ×ÓŗĖÄŚµÄÖŹ×ÓŹżŹĒDŌ×ÓŗĖĶāµē×ÓŹżµÄ2±¶”£¾Ż“Ė£¬Ēė»Ų“š£ŗ
£Ø1£©FŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ____________________________”£
£Ø2£©ÓÉA”¢C”¢D”¢F°“8:2:4:1Ō×ÓøöŹż±Č×é³ÉµÄ»ÆŗĻĪļ¼×ÖŠŗ¬ÓŠµÄ»Æѧ¼üĄąŠĶĪŖ____________£»¼×ČÜŅŗÖŠø÷Ąė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ________________£ØÓĆĄė×ÓÅØ¶Č·ūŗűķŹ¾£©”£
£Ø3£©»ÆŗĻĪļŅŅÓÉA”¢C×é³ÉĒŅĻą¶Ō·Ö×ÓÖŹĮæĪŖ32£»»ÆŗĻĪļ±ūÓÉA”¢D×é³ÉĒŅ·Ö×ÓÄŚµē×Ó×ÜŹżÓėŅŅ·Ö×ÓÄŚµē×Ó×ÜŹżĻąµČ£»ŅŅÓė±ūµÄ·“Ó¦æÉÓĆÓŚ»š¼ż·¢Éä£Ø·“Ó¦²śĪļ²»ĪŪČ¾“óĘų£©£¬ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________________”£
£Ø4£©ÓÉA”¢D”¢E”¢F×é³ÉµÄ»ÆŗĻĪļ¶”ÄÜÓėĮņĖį·“Ó¦²¢·Å³ö“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壬Ōņ¶”µÄ»ÆѧŹ½ĪŖ£ß£ß£ß£ß£ß£ß£ß£ß£»ŹµŃé²āµĆ¶”ČÜŅŗĻŌČõĖįŠŌ£¬ÓÉ“ĖÄćÄÜµĆ³öµÄ½įĀŪŹĒ___________________”£
£Ø5£©ÓÉB”¢A°“1:4Ō×ÓøöŹż±Č×é³ÉµÄ»ÆŗĻĪļĪģÓėDµÄ³£¼ūĘųĢ¬µ„ÖŹ¼°NaOHČÜŅŗ¹¹³ÉŌµē³Ų
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀ±ķĪŖŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö,Ēė²ĪÕÕŌŖĖŲ¢Ł”«¢įŌŚ±ķÖŠµÄĪ»ÖĆ,ÓĆ»ÆѧÓĆÓļ»Ų“šĻĀĮŠĪŹĢā:
(1)ŅŃÖŖÓÉ¢Ł”¢¢Ū”¢¢ÜČżÖÖŌŖĖŲ×é³ÉµÄijÖÖ³£¼ū»ÆŗĻĪļµÄĖ®ČÜŅŗ³ŹĖįŠŌ,Ōņ·ūŗĻøĆĢõ¼žµÄ»ÆŗĻĪļµÄ»ÆѧŹ½æÉÄÜĪŖ (ÖĮÉŁŠ“Į½ÖÖ)”£
(2)ÓɱķÖŠ¢Ł”¢¢ÜĮ½ÖÖŌŖĖŲ°“Ō×ÓøöŹż±ČĪŖ1”Ć1¹¹³ÉµÄ»ÆŗĻĪļµÄĻ”ČÜŅŗŅ×±»“߻Ʒֽā,Ķس£Ź¹ÓĆµÄ“ß»Æ¼ĮĪŖ (ĢīŠņŗÅ)”£
aӢMnO2 bӢFeCl3 cӢNa2SO3 dӢKMnO4
(3)ĻĀĶ¼ÖŠA”«FŹĒÓɲæ·ÖÉĻ±ķÖŠŌŖĖŲ×é³ÉµÄµ„ÖŹ»ņ»ÆŗĻĪļ,ĘäÖŠA”¢B”¢CĪŖµ„ÖŹ,×Ŗ»Æ¹ŲĻµČēĻĀ: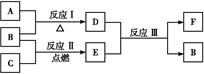
¢ń.ČōBĪŖ»ĘÉ«¹ĢĢå,AĪŖŌ×Ó°ė¾¶×īŠ”µÄŌ×Ó×é³ÉµÄµ„ÖŹ”£
¢ŁFµÄµē×ÓŹ½ĪŖ ”£
¢ŚŹµŃé²āµĆĘšŹ¼²Ī¼Ó·“Ó¦µÄBŗĶ×īŗóÉś³ÉµÄBÖŹĮæĻąµČ,ŌņĘšŹ¼²Ī¼Ó·“Ó¦µÄAŗĶCµÄĪļÖŹµÄĮæÖ®±ČŹĒ ”£
¢ņ.ČōDĪŖµ»ĘÉ«¹ĢĢå,ŃęÉ«·“Ó¦ĪŖ»ĘÉ«,×é³ÉCµÄŌŖĖŲµÄŌ×Ó×īĶā²ćµē×ÓŹżŹĒÄŚ²ćµē×ÓŹżµÄ2±¶”£
¢ŁĻĀĮŠ¹ŲÓŚDµÄĖµ·ØÕżČ·µÄŹĒ (Ģī×ÖÄø)”£
a.ÄÜÓėĖ®·¢Éś»ÆŗĻ·“Ó¦
b.¼ČÓŠŃõ»ÆŠŌ,ÓÖÓŠ»¹ŌŠŌ
c.¼Čŗ¬Ąė×Ó¼ü,ÓÖŗ¬·Ē¼«ŠŌ¹²¼Ū¼ü
d.ŹĒŅ»ÖÖ¼īŠŌŃõ»ÆĪļ
¢ŚÓƶčŠŌµē¼«½«FµÄ±„ŗĶČÜŅŗ½ųŠŠµē½ā,ŌņŃō¼«·“Ó¦Ź½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶ĢÖÜĘŚŌŖĖŲX”¢Y”¢Z”¢L”¢M”¢QµÄŌ×Ó°ė¾¶ŗĶÖ÷ŅŖ»ÆŗĻ¼Ū¼ūĻĀ±ķ
| ŌŖĖŲ“śŗÅ | X | Y | Z | L | M | Q |
| Ō×Ó°ė¾¶/nm | 0£®160 | 0£®143 | 0£®102 | 0£®099 | 0£®077 | 0£®074 |
| Ö÷ŅŖ»ÆŗĻ¼Ū | +2 | +3 | +6”¢-2 | +7”¢-1 | +4”¢-4 | -2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĖłŹ¾ĪŖŃŖŗģµ°°×ŗĶ¼”ŗģµ°°×µÄ»īŠŌ²æ·Ö”Ŗ”ŖŃŖŗģĖŲµÄ½į¹¹Ź½”£
»Ų“šĻĀĮŠĪŹĢā:
(1)ŃŖŗģĖŲÖŠŗ¬ÓŠC”¢H”¢O”¢N”¢FeĪåÖÖŌŖĖŲ,C”¢N”¢OČżÖÖŌŖĖŲµÄµŚŅ»µēĄėÄÜÓÉŠ”µ½“óµÄĖ³ŠņŹĒ””””””””””””””””,Š“³ö»łĢ¬FeŌ×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½:”””””””””””””””””£ 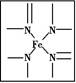
(2)ŃŖŗģĖŲÖŠNŌ×ÓµÄŌӻƷ½Ź½ĪŖ””””””””,ŌŚČēĶ¼µÄ·½æņÄŚÓĆ”°”ś”±±ź³öFe2+µÄÅäĪ»¼ü”£
(3)ĢśÓŠ¦Ä”¢¦Ć”¢¦ĮČżÖÖĶ¬ĖŲŅģŠĪĢå,¦Ć¾§Ģ徧°ūÖŠĖłŗ¬ÓŠµÄĢśŌ×ÓŹżĪŖ””””””””,¦Ä”¢¦ĮĮ½ÖÖ¾§°ūÖŠĢśŌ×ÓµÄÅäĪ»ŹżÖ®±ČĪŖ”””””””””£ 
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
X”¢Y”¢Z”¢WŹĒŌŖĖŲÖÜĘŚ±ķĒ°ĖÄÖÜĘŚÖŠµÄ³£¼ūŌŖĖŲ£¬ĘäĻą¹ŲŠÅĻ¢ČēĻĀ±ķ£ŗ
| ŌŖĖŲ | Ļą ¹Ų ŠÅ Ļ¢ |
| X | X×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļÓėĘųĢ¬Ēā»ÆĪļæÉŅŌŠĪ³ÉŅ»ÖÖŃĪ |
| Y | µ„ÖŹŹĒĮ¼ŗƵİėµ¼Ģå²ÄĮĻ£¬¹ć·ŗÓ¦ÓĆÓŚ¹āµēŠÅĻ¢ĮģÓņ |
| Z | ZµÄŅ»ÖÖŗĖĖŲÖŹĮæŹżĪŖ27£¬ÖŠ×ÓŹżĪŖ14 |
| W | ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļŹĒŅ»ÖÖ²»ČÜÓŚĖ®µÄĄ¶É«¹ĢĢå |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com