
ijͬѧ��FeCl3���������Լ�FeSO4�����ȶ��Խ�������̽����
��1����������FeSO4���·ֽ⣬��Ӧ����ʽΪ�� 2FeSO4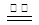 Fe2O3+SO2��+SO3����
Fe2O3+SO2��+SO3����
����˫���ű�����·ֽ�FeSO4�Ʊ�Fe2O3��Ӧ�е���ת�Ƶķ������Ŀ ��
��Ϊ�ռ�SO3����֤SO2��Ӧ����������ͨ����û�� �е�U�ܡ�ϴ��ƿ�е� ��NaOH��Һ��
��2��ʵ��̽��Fe3+�������ԣ���FeCl3��Һ��ͨ��һ������SO2���壬��Һ�ɻ�ɫ��Ϊdz��ɫ��
��dz��ɫ��Һ��һ�����ڵ�������H+��Cl-�� �����ܴ��ڵ����� (��д���)��
A��Fe3+ B��Fe2+ C��SO42- D��H2SO3
��Ϊȷ�Ͽ��ܴ��ڵ�����Ӧѡ����Լ��� (��д���)��
A��ϡ���� B��NaOH��Һ C��KSCN��Һ D��Ʒ����Һ
�� ��2�֣�
��2�֣�
�� ��ˮ����Ʒ����Һ��������ȷ�𰸣���2�֣���
��2����BC��2�֣��� AD��2�֣���ѡ��һ����1�֣���ѡ0�֣�
��CD��2�֣���ѡ��һ����1�֣���ѡ0�֣�
���������������1��FeSO4���·ֽ�����ΪSO2��SO3���ռ�SO2���ȷ����SO3������SO3�ܷе�߳�����ΪҺ�壬���ñ�ˮ��ȴ���ɵõ�SO3����ʹ�����SO2���壻����SO2һ����Ʒ���Լ�����2����Һ�ɻ�ɫ��Ϊdz��ɫ��˵��Fe3+��SO2���巴Ӧ����Fe2+��SO4����������δ��Fe3+��Ӧ��SO2��ˮ��Ӧ����H2SO3��������������Ϊδ��Ӧ���Fe3+���������������ֱ���Ʒ����Һ��KSCN��Һ��
���㣺����������ԭ��Ӧ����ԭ���й����⡣


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С�齫һ����������·�徭Ũ�����ϡ���ᴦ����õ�һ�����Һ,���к���Cu2+��Fe2+��Fe3+��Al3+�Ƚ�������,��������������������Էֱ���ȡCuSO4��5H2O�����AlCl3
��Һ: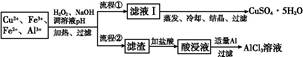
��֪:��ؽ������ӿ�ʼ��������ȫ����ʱ��pH��ΧΪ:
| ���� | Fe3+ | Fe2+ | Al3+ | Cu2+ |
| pH��Χ | 2.2��3.2 | 5.5��9.0 | 4.1��5.0 | 5.3��6.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺,���������ʵ��ش�����:
(1)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и�������,�����д��ԵĹ���Y,��Y���ڹ����������Һ�к��еĴ�������������������
(2)ij��Һ����Mg2+��Fe2+��Al3+��Cu2+����������,�����м��������NaOH��Һ��,����,�������������ղ������պ�Ĺ���Ͷ�������ϡ������,������Һ��ԭ��Һ���,��Һ�д������ٵ�����������������
A.Mg2+ B.Fe2+ C.Al3+ D.Cu2+
(3)����������Ҫ��ҵ����,�÷���м�Ʊ�������������:
�ش���������:
�ٲ������������������,���������������������
��д���ڿ���������FeCO3�Ļ�ѧ����ʽ������������������������������
(4)��Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��
a.��ȡ2.850 g�̷�(FeSO4��7H2O)��Ʒ,�ܽ�,��250 mL����ƿ�ж���;
b.��ȡ25.00 mL������Һ������ƿ��;
c.�������ữ��0.010 00 mol��L-1 KMnO4��Һ�ζ����յ�,����KMnO4��Һ�����ƽ��ֵΪ20.00 mL��
��ʵ��ǰ,����Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL,����ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι���,������������
��ijͬѧ��Ƶ����еζ���ʽ,�����������������(�гֲ�����ȥ)(����ĸ���) 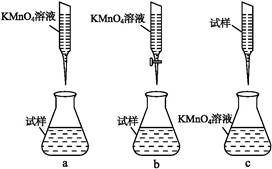
��д���ζ������з�Ӧ�����ӷ���ʽ:��������������������������������������
�ܼ���������Ʒ��FeSO4��7H2O����������Ϊ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����ĵ�������к���ͭ�����Ƚ��������Ϊʵ����Դ�Ļ������ò���Ч��ֹ������Ⱦ��������¹������̣�
��1����������H2O2��Ŀ���� ����pH�����м�����Լ������ ���ѧʽ����ʵ���ҽ��й��˲������õ��IJ��������� ��
��2�����CuSO4��Һ��ԭ���� ����CuSO4��Һ�м���һ������NaCl��Na2SO3���������ɰ�ɫ��CuCl������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3����ȡ���Ʊ���CuCl��Ʒ0.2500g����һ������0.5mol��L-1FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000mol��L-1��Ce��SO4��2��Һ�ζ��������յ�ʱ����Ce��SO4��2��Һ25.00mL���йصĻ�ѧ��ӦΪ��Fe3����CuCl��Fe2����Cu2����Cl����Ce4����Fe2����Fe3����Ce3���������CuCl��Ʒ���������� ��
��4��Fe3+����ˮ�ⷴӦFe3����3H2O Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ������ʽΪ ��
Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ������ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
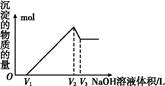
��20 mL ijŨ�ȵ�AlCl3��Һ�е���2 mol��L��1��NaOH��Һʱ�����õij������������NaOH��Һ�����֮��Ĺ�ϵ��ͼ��ʾ��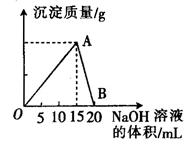
��1��ͼ��A���ʾ��������______________��
��2������������______________g��
��3��B���ʾ��������______________��
��4������AlCl3��Һ�����ʵ���Ũ����______________��
��5�������ó�����Ϊ0.39��ʱ����ȥNaOH��Һ�������_____ mL ��_______ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����֣���Ҫ�ɷ�ΪFe����Ʒ�к�������ͭ�ȣ������ɷֺ��ԣ�,Ϊ�˲ⶨ�úϽ������ĺ���,����������¹������̣�
��1����ҺC�����ʺ���_ _��д��ѧʽ����
��2�������֤��ҺA�к�Fe2+��������Fe3+____ _____��
��3��������Fe2O3�������������Ϊb g���������Ʒ����Ԫ�����������ı���ʽΪ���ú�a��b��ʽ�ӱ�ʾ��___ __��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���Ѱ۲����ķ�Һ�к��д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4����������ⷨ������Ѫ�������������������������£�
��֪��TiOSO4������ˮ����ˮ�е���ΪTiO2+��SO42-����ش��������⣺
��1��д��TiOSO4ˮ����������H4TiO4�����ӷ���ʽ ��������м���������м��Ŀ���� ��
��2����ҵ����H4TiO4���Ƶ��Ѱ�TiO2��TiO2ֱ�ӵ�ԭ�������ŷ��������� ��һ�ֽ��Ƚ��ķ����������Ϊ���ڵ�CaCl2��ԭ����ͼ��ʾ�������ĵ缫��ӦΪ_______________��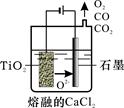
��3������ڵ����ӷ���ʽ�� �����ø���Ʒ��Ҫ ��__________���ѧʽ����
��4������ܵĽᾧ�����б������һ������նȣ�ԭ���� ��
��5�����������ϩ�����в���ϳɣ�
�����ϳ�·�ߵ��ܲ���Ϊ60%��������̼��������Ӧת��Ϊ������������IJ���Ϊ90%��������468 kg�����������壨M��234 g/mol����Ҫ��״���µ���ϩ m3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ú���Al2O3������Fe2O3��SiO2���������Ʊ���ˮ������Һ��ۺ����������������������£����ֲ����������ԣ���
I�����������м������H2SO4���ȡ����衢���ˡ�
II������Һ�м���һ������FeSO4��7H2O��˫��ˮ��
III������Һ�м���Ca(OH)2���壬������Һ��pHԼΪ1�����ˡ�
IV�������ȶ��������ȣ��õ���Ʒ��
��1��Fe2O3��H2SO4��Ӧ�����ӷ���ʽ��___________��
��2������I�й��˵õ��������ɷ���________���ѧʽ����
��3������I ��H2SO4��Ũ���뷴Ӧ�¶Ȼ�Ӱ���������Ľ����ʡ�������ͼ����������I ��H2SO4Ũ�ȵ����˷�Χ��__________����Ӧ�������¶���_________��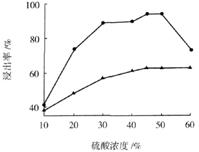

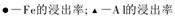
��4������II������n(Fe3+)�����ӷ���ʽ��_________��
��5������III�õ���ʽ��������[AlFe(OH)n(SO4)m]����Һ������II��Ӧ����n(Fe3+)��
n(Al3+)�sn(Fe3+)= ��
��6���о�������Һ��ۺ����������Ĵ���Խ�ߣ���ˮЧ��Խ�á���֪��
һЩ������20��ʱ���ܽ��
| ���� | Ca(OH)2 | CaSO4 | Na2SO4 |
| �ܽ��/g | 0.153 | 0.258 | 19.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.�ִ���ҵ�����Ȼ���Ϊԭ���Ʊ�������ֹ����������£�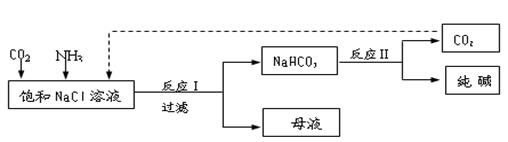 k+s-5#
k+s-5#
��֪NaHCO3�ڵ������ܽ�Ƚ�С����Ӧ��Ϊ��NaCl+CO2+NH3+H2O NaHCO3��+NH4Cl������ĸҺ�����ַ������£�
NaHCO3��+NH4Cl������ĸҺ�����ַ������£�
��1����ĸҺ�м���ʯ���飬�ɽ�����________ѭ�����á�
��2����ĸҺ��ͨ��NH3������ϸС��ʳ�ο��������£��ɵõ�NH4Cl���塣��д��ͨ��NH3���ܽ�Ƚ�С����ʽ̼����ת��Ϊ�ܽ�Ƚϴ��̼���ε����ӷ���ʽ _____________________________��
��.ij��ѧС��ģ�⡰�����Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ����ȡNaHCO3��Ȼ���ٽ�NaHCO3�Ƴ�Na2CO3��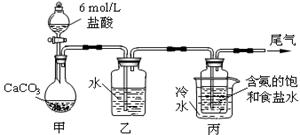
��1��װ�ñ�����ˮ�������� ��
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ�������_______��ϴ�ӡ����ա�NaHCO3ת��ΪNa2CO3�Ļ�ѧ����ʽΪ ��
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1 min��NaHCO3 ��Ʒ����ɽ���������̽����ȡ������t1 min��NaHCO3��Ʒ29.6 g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��衣��������ļ��룬��Һ���й����ӵ����ʵ����ı仯��ͼ��ʾ��������a��Ӧ����Һ�е�������___________�������ӷ�����ͬ��������c��Ӧ����Һ�е�������___________������Ʒ��NaHCO3��Na2CO3�����ʵ���֮���� ��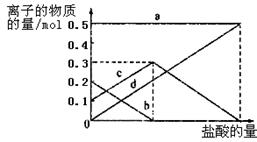
��4����ȡ21.0 g NaHCO3���壬������t2 rnin��ʣ����������Ϊl4.8 g������Ѵ�ʣ�����ȫ�����뵽200 mL 2 mol?L��1�������У����ַ�Ӧ����Һ��H+ �����ʵ���Ũ��Ϊ____________������Һ����仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com