
���� ��1��Ũ������лӷ��ԣ��ӷ�����HCl�����ʯ�ҷ�Ӧ��
��2�����̼�����Ƶ����������÷���ʽ2NaHCO3+H2SO4�TNa2SO4+2H2O+2CO2�������̼�����Ƶ����������ڼ�ʯ����ͬʱ���ն�����̼��ˮ��������̼�����������ˮҲ����ʯ�����գ���̼�����Ƶ�����ƫС$\frac{1.4}{a}$��
��3���ٷ���һ��̼�����ƿ���û����ȫ�ֽ⣬Ӱ��ⶨ�����
�ڷ������м�ʯ�Ҽ������ն�����̼��������ˮ��Ӱ��ⶨ�����
��4������һ����Ҫ��ּ��ȣ�����������ͨ��װ��Ũ�����ϴ��ƿ���
��� �⣺��1��Ũ������лӷ��ԣ������ʯ�ҷ�Ӧ������Ũ���ᣬ���ʹ��ʯ�����ӵ��������ʲ���ѡ��Ũ���ᣬ
�ʴ�Ϊ�����ܣ��ӷ����Ȼ���ͬʱ����ʯ�����գ�
��2����̼�����Ƶ�����Ϊx�������ʯ�����ص�����ȫ��Ϊ������̼��������
2NaHCO3+H2SO4�TNa2SO4+2H2O+2CO2��
2��84 2��44
x 6.6g
$\frac{2��84}{x}$=$\frac{2��44}{6.6g}$����ã�x=4.2g��
��Ϊ��ʯ����ͬʱ���ն�����̼��ˮ��������̼�����������ˮҲ����ʯ�����գ��ʶ�����̼ʵ������С��6.6g�����̼�����Ƶ�ʵ������С��4.2g��������������С��$\frac{1.4}{a}$��
�ʴ�Ϊ����$\frac{1.4}{a}$��
��3���ٷ���һ ֻ�ǽ���������һ��ʱ�䣬����֪̼�������Ƿ���ȫ�ֽ⣬�ʲ�һ��ȷ��
�ʴ�Ϊ����ȷ��NaHCO3����δ��ȫ�ֽ⣻
�ڷ����������ڼ�ʯ�Ҽ������ն�����̼��������ˮ�����������������Բⶨ�����ȷ��
�ʴ�Ϊ����ȷ��H2O�ڼ���ʱ����̬��ͬʱ�����գ�����ƫ��
��4������һ�п��Ը�Ϊ���������岻�ټ���ʱΪֹ���������п�����ͨ��װ��Ũ�����ϴ��ƿ����Ȼ�����ü�ʯ������CO2��
�ʴ�Ϊ������һ��Ϊ���������岻�ټ���ʱΪֹ��������ͨ��װ��Ũ�����ϴ��ƿ����Ȼ�����ü�ʯ������CO2����
���� ���⿼���˻�ѧʵ�鷽�������ۣ���Ŀ�Ѷ��еȣ���ȷʵ��Ŀ�ġ�ʵ��ԭ��Ϊ���ؼ�����3����4��Ϊ�ѵ㡢�״��㣬ע�����ջ�ѧʵ�鷽�����۵�ԭ���������������ѧ���Ļ�ѧʵ��������


 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ | |
| B�� | ʹ���ȵĴ�����Һȥ������ | |
| C�� | ʵ��������FeCl3��Һʱ��������ϡ���� | |
| D�� | ʵ����������ʱ����CuSO4�ɼӿ췴Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 $��_{��}^{ŨH_{2}SO_{4}}$
$��_{��}^{ŨH_{2}SO_{4}}$ +H2O��
+H2O�� ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �øɱ��˹����� | B�� | װ����ʯ��ˮ��ƿ������һ���Ĥ | ||
| C�� | ��Ʒ���������ɫ�Ĺ� | D�� | ���ֱ�ը |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 100 mL | B�� | 200 mL | C�� | 300 mL | D�� | 400 mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
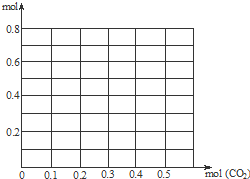 ����0.2mol NaOH��0.1mol Ca��OH��2�Ļ����Һ�г����ȶ���ͨ��CO2����0.5mol������CO2����Ϊ�����꣬����Һ�����ӵ�����Ϊ�����꣬��������������CO2�������仯������ͼ��������������ʵĵ�����ε�ˮ�⣩
����0.2mol NaOH��0.1mol Ca��OH��2�Ļ����Һ�г����ȶ���ͨ��CO2����0.5mol������CO2����Ϊ�����꣬����Һ�����ӵ�����Ϊ�����꣬��������������CO2�������仯������ͼ��������������ʵĵ�����ε�ˮ�⣩�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͭ������֡�Ӳ�����ǺϽ� | |
| B�� | ͭ�������γ����ܵ�����Ĥ | |
| C�� | ͭ��O2��Ӧ�����ɺ�ɫ��Cu2O | |
| D�� | CuSO4•5H2O��һ�ֻ������Ⱥ��Ϊ��ɫ��ĩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2mol SO2��1mol O2��һ�������³�ַ�Ӧ�����û������ķ�����С��2NA | |
| B�� | ��1mol CH3COONa������CH3COOH�γɵ�������Һ�У�CH3COO-��ĿΪNA�� | |
| C�� | ���³�ѹ�£�14g��N2��CO��ɵĻ�����庬�е�ԭ����ĿΪNA | |
| D�� | ��״���£�6.72L NO2��ˮ��ַ�Ӧת�Ƶĵ�����ĿΪ0.2NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com