
��6�֣�(1)��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γ�(���)1 mol��ѧ��ʱ�ͷ�(������)����������֪��N��N���ļ�����948.9 kJ/mol��H��H���ļ�����436.0 kJ/mol����N2��H2�ϳ�1 mol NH3ʱ�ɷų�46.2 kJ����������N��H���ļ�����_______________________��
(2)��˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ����ʽ��
Fe2O3(s)+3CO(g) = 2Fe(s)+3CO2(g) ��H����24.8 kJ/mol ��
3Fe2O3(s)+CO(g) = 2Fe3O4(s)+CO2(g) ��H����47.2 kJ/mol ��
Fe3O4(s)+CO(g) = 3FeO(s)+CO2(g) ��H��+640.5 kJ/mol ��
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ����ʽ______________________________��
(3)��֪�����Ȼ�ѧ����ʽ��
C(s)��O2(g)=CO2(g) ��H�� �D 393.5kJ/mol
2H2(g)��O2(g)=2H2O(g) ��H�� �D 483.6kJ/mol
����̿�ۺ�H2��ɵ���������0.2mol��ʹ����O2����ȫȼ�գ����ų�63.53kJ����������̿����H2�����ʵ���֮���� .
 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
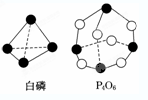 ��1����ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���ѧ���ļ������γɣ����1mol ��ѧ��ʱ�ͷţ������գ�����������֪����P4O6�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�E��P-P��=198kJ/mol��E��P-O��=360kJ/mol��E��O�TO��=498kJ/mol����ӦP4�����ף�ȼ������P4O6���Ȼ�ѧ����ʽΪ
��1����ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���ѧ���ļ������γɣ����1mol ��ѧ��ʱ�ͷţ������գ�����������֪����P4O6�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�E��P-P��=198kJ/mol��E��P-O��=360kJ/mol��E��O�TO��=498kJ/mol����ӦP4�����ף�ȼ������P4O6���Ȼ�ѧ����ʽΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���֪�Ͽ�1mol���л�ѧ��ʱ��Ҫ���յ������ֱ�Ϊ��P-P 198kJ��P-O 360kJ��O=O 498kJ
��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���֪�Ͽ�1mol���л�ѧ��ʱ��Ҫ���յ������ֱ�Ϊ��P-P 198kJ��P-O 360kJ��O=O 498kJ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣�(1)��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γ�(���)1 mol��ѧ��ʱ�ͷ�(������)����������֪��N��N���ļ�����948.9 kJ/mol��H��H���ļ�����436.0 kJ/mol����N2��H2�ϳ�1 mol NH3ʱ�ɷų�46.2 kJ����������N��H���ļ�����_______________________��
(2)��˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ����ʽ��
Fe2O3(s)+3CO(g) = 2Fe(s)+3CO2(g)��H����24.8 kJ/mol ��
3Fe2O3(s)+CO(g) = 2Fe3O4(s)+CO2(g)��H����47.2 kJ/mol ��
Fe3O4(s)+CO(g) = 3FeO(s)+CO2(g)��H��+640.5 kJ/mol ��
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ����ʽ______________________________��
(3)��֪�����Ȼ�ѧ����ʽ��
C(s)��O2(g)=CO2(g) ��H�� �D393.5kJ/mol
2H2(g)��O2(g)=2H2O(g) ��H�� �D483.6kJ/mol
����̿�ۺ�H2��ɵ���������0.2mol��ʹ����O2����ȫȼ�գ����ų�63.53kJ����������̿����H2�����ʵ���֮���� .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ����������һ�з�У�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��6�֣�(1)��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γ�(���)1 mol��ѧ��ʱ�ͷ�(������)����������֪��N��N���ļ�����948.9 kJ/mol��H��H���ļ�����436.0 kJ/mol����N2��H2�ϳ�1 mol NH3ʱ�� �ų�46.2 kJ����������N��H���ļ�����_______________________��
�ų�46.2 kJ����������N��H���ļ�����_______________________��
(2)��˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ����ʽ��
Fe2O3(s)+3CO(g) = 2Fe(s)+3CO2(g) ��H����24.8 kJ/mol ��
3Fe2O3(s)+CO(g) = 2Fe3O4(s)+CO2(g) ��H����47.2 kJ/mol ��
Fe3O4(s)+CO(g) = 3FeO(s)+CO2(g) ��H��+640 .5 kJ/mol ��
.5 kJ/mol ��
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ����ʽ______________________________��
(3)��֪�����Ȼ�ѧ����ʽ��
C(s)��O2(g)=CO2 (g) ��H�� �D 393.5kJ/mol
(g) ��H�� �D 393.5kJ/mol
2H2(g)��O2(g )=2H2O(g) ��H�� �D 483.6kJ/mol
)=2H2O(g) ��H�� �D 483.6kJ/mol
����̿�ۺ�H2��ɵ���������0.2mol��ʹ����O2����ȫȼ�գ����ų�63.53kJ����������̿����H2�����ʵ���֮���� .
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com