
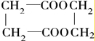
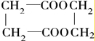
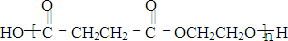
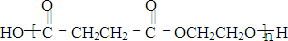
| Cu |
| ”÷ |
| “߻ƼĮ |
| ”÷ |
| Cu |
| ”÷ |
| “߻ƼĮ |
| ”÷ |
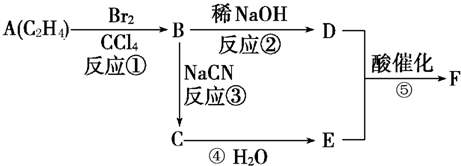
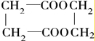 £¬½įŗĻ¶ŌÓ¦ÓŠ»śĪļµÄŠŌÖŹŗĶĢāÄæŅŖĒóæɽā“šøĆĢā£®
£¬½įŗĻ¶ŌÓ¦ÓŠ»śĪļµÄŠŌÖŹŗĶĢāÄæŅŖĒóæɽā“šøĆĢā£®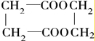 £¬
£¬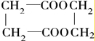 £¬
£¬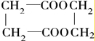 £»
£»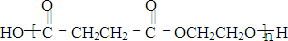 £¬
£¬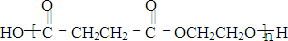 £»
£»| Cu |
| ”÷ |
| “߻ƼĮ |
| ”÷ |
| Cu |
| ”÷ |
| “߻ƼĮ |
| ”÷ |


 ĆĻ½ØĘ½Š”ѧ¹ö¶Æ²āŹŌĻµĮŠ“š°ø
ĆĻ½ØĘ½Š”ѧ¹ö¶Æ²āŹŌĻµĮŠ“š°ø »ĘøŌĢģĢģĮ·æŚĖćĢāæØĻµĮŠ“š°ø
»ĘøŌĢģĢģĮ·æŚĖćĢāæØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
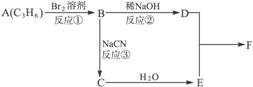
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
²śĪļ·Ö×Ó±ČŌ»ÆŗĻĪļ·Ö×Ó¶ąĮĖŅ»øöĢ¼Ō×Ó£¬Ōö³¤ĮĖĢ¼Į“”£Ēėøł¾ŻÓŅ±ßæņĶ¼»Ų“šĪŹĢā£ŗ
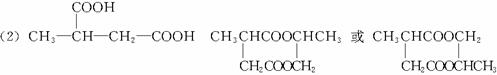
F·Ö×ÓÖŠŗ¬ÓŠ8øöŌ×Ó×é³ÉµÄ»·×“½į¹¹”£
(1)·“Ó¦¢Ł¢Ś¢ŪÖŠŹōÓŚČ”“ś·“Ó¦µÄŹĒ___________(Ģī·“Ó¦“śŗÅ)”£
(2)Š“³ö½į¹¹¼ņŹ½£ŗE___________£¬F___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
²śĪļ·Ö×Ó±ČŌ»ÆŗĻĪļ·Ö×Ó¶ąĮĖŅ»øöĢ¼Ō×Ó£¬Ōö³¤ĮĖĢ¼Į“”£Ēėøł¾ŻŅŌĻĀæņĶ¼Ķź³ÉĻĀĮŠĪŹĢā£ŗ
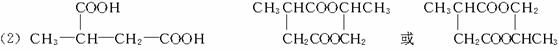
F·Ö×ÓÖŠŗ¬ÓŠ8øöŌ×Ó×é³ÉµÄ»·×“½į¹¹”£
£Ø1£©·“Ó¦¢Ł¢Ś¢ŪÖŠŹōÓŚČ”“ś·“Ó¦µÄŹĒ____________(Ģī·“Ó¦“śŗÅ)”£
£Ø2£©Š“³ö½į¹¹¼ņŹ½£ŗE____________£¬F____________”£
²éæ““š°øŗĶ½āĪö>>
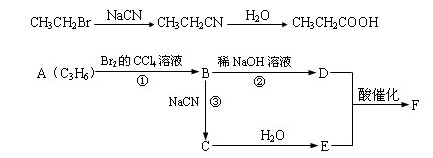
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖäåŅŅĶéøśĒč»ÆÄĘ·“Ó¦ŗóŌŁĖ®½āæÉŅŌµĆµ½±ūĖį£ŗ
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
²śĪļ·Ö×Ó±ČŌ»ÆŗĻĪļ·Ö×Ó¶ąĮĖŅ»øöĢ¼Ō×Ó£¬Ōö³¤ĮĖĢ¼Į“”£Ēėøł¾ŻÓŅ±ßæņĶ¼»Ų“šĪŹĢā£ŗ
F·Ö×ÓÖŠŗ¬ÓŠ8øöŌ×Ó×é³ÉµÄ»·×“½į¹¹”£
(1)·“Ó¦¢Ł¢Ś¢ŪÖŠŹōÓŚČ”“ś·“Ó¦µÄŹĒ___________(Ģī·“Ó¦“śŗÅ)”£
(2)Š“³ö½į¹¹¼ņŹ½£ŗ£Å___________£¬£Ę___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖäåŅŅĶéøśĒč»ÆÄĘ·“Ó¦ŗóŌŁĖ®½āæÉŅŌµĆµ½±ūĖį
²śĪļ·Ö×Ó±ČŌ»ÆŗĻĪļ·Ö×Ó¶ąĮĖŅ»øöĢ¼Ō×Ó£¬Ōö³¤ĮĖĢ¼Į“”£
Ēėøł¾ŻŅŌĻĀæņĶ¼»Ų“šĪŹĢā”£Ķ¼ÖŠF·Ö×ÓÖŠŗ¬ÓŠ8øöŌ×Ó×é³ÉµÄ»·×“½į¹¹”£
£Ø1£©·“Ó¦¢Ł¢Ś¢ŪÖŠŹōÓŚČ”“ś·“Ó¦µÄŹĒ______________£ØĢī·“Ó¦“śŗÅ£©”£
£Ø2£©Š“³ö½į¹¹¼ņŹ½£ŗE___________£¬F______________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com