
 X��Y��Z��W��T�Ƕ����ڱ��е�5������Ԫ�أ�X��̬ԭ�Ӻ���L��p����������s��������Y��һ�ֺ��ص�������Ϊ19������������������1��T-���Ӻ�����������ԭ�ӵĺ����������ͬ��X��Y��Z��W�ĵ�һ�����������ǵĺ˵�����Ĺ�ϵ��ͼ��ʾ��
X��Y��Z��W��T�Ƕ����ڱ��е�5������Ԫ�أ�X��̬ԭ�Ӻ���L��p����������s��������Y��һ�ֺ��ص�������Ϊ19������������������1��T-���Ӻ�����������ԭ�ӵĺ����������ͬ��X��Y��Z��W�ĵ�һ�����������ǵĺ˵�����Ĺ�ϵ��ͼ��ʾ��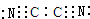 ��
��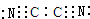 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Mg��HCO3��2��Һ�м�������� NaOH��Һ��Mg2++2HCO3-+4OH-=Mg��OH��2��+2CO32-+2H2O |
| B���������ᱵ�����м���ϡ���3BaSO3+2H++2NO3-=3BaSO4��+2NO��+H2O |
| C����������Һ�еμӹ���ϡ���[Ag��NH3��2]++2H+=Ag++2NH4+ |
| D����NH4HSO4ϡ��Һ����μ���Ba��OH��2ϡ��Һ��SO42-�պó�����ȫ��Ba2++2OH-+NH4++H++SO42-=BaSO4��+NH3?H2O+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
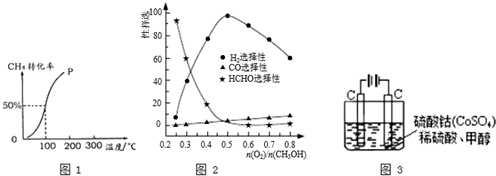
| 1 |
| 2 |
| c(H2) |
| c(CH3OH) |
| n(O2) |
| n(CH3OH) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Һ��SO42-��Ũ����0.3 mol/L |
| B����Һ��һ������A13+��NH4+ |
| C��һ��������Mg2+�����ܴ���A13+ |
| D��һ������Cl- ���ܺ�CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴˣ��ش����⣺
��ͼ��ѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴˣ��ش����⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com