
��������������Ⱦ��Ϊ���أ����������������ü�����⡣
(1)���������һ�������ȼҵ��Ʒ������������������ķ������������£�
(��)����SO2�ķ���ͨ���ⱥ��ʳ��ˮ�����õ�����Һ�У���NaHSO3��Һ��
(��)����ⱥ��ʳ��ˮ�������巴Ӧ���Ƶ����ᡣ
(��)���������NaHSO3��Һ�У���Ӧ���õ���SO2������գ����ɵ�NaClѭ�����á�
��д������(��)��Ӧ�Ļ�ѧ����ʽ�� ��
��д������(��)�е�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ�� ��
��д������(��)��Ӧ�����ӷ���ʽ�� ��
(2)����ѧ���������Fe2����Fe3�������ӵĴ����ã������½�SO2������SO42-��ʵ��SO2�Ļ������á�ij�о���ѧϰС��ݴ���������·�������ʵ���������²ⶨת������SO2������SO42-��ת���ʡ�
�ٸ�С�������ͼװ����ʵ���Ҳⶨģ��������SO2�����������X��Һ������ ��(��д���)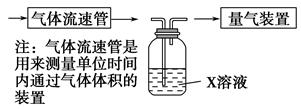
| A����ĵ�����Һ | B�����Ը��������Һ |
| C������������Һ | D���Ȼ�����Һ |
 ��ת���ʣ���֪�������٣�����ⶨ�������� �� ��
��ת���ʣ���֪�������٣�����ⶨ�������� �� ��  �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������һ����Ҫ�Ĺ�ҵԭ�ϡ���ҵ�����÷�Ӧ��3Cl2+8NH3=N2+6NH4Cl��������ܵ��Ƿ�©��������˵����ȷ����
| A�����ܵ�©�������ͻ�������� | B���÷�Ӧ�����˰����Ļ�ԭ�� |
| C���÷�Ӧ���ڸ��ֽⷴӦ | D������6molNH4Cl��18mol����ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(10��)(I) �����к��е�Ԫ�أ�ijУ�о���ѧϰС�����������ʵ�鲽������ȡ�⣺
������Һ�У��μӼ��������������˫��ˮ �ڽ������ճɻң�����м�ˮ�����Ƚ���
�ۼ�CC14������ �ܹ��� �ݷ�Һ��
��1�������IJ���˳��Ϊ ��
��2���������Ҫ�õ��IJ�������Ϊ ���ò�����I2�IJ����� ��
(II)��ij����Fe2+��I����Br������Һ�л���ͨ��������������Һ�и������ӵ����ʵ����仯��ͼ��ʾ��
��3��AB�α�ʾ ���ӵļ��١�
��4��n(Cl2)=2molʱ����Һ�з�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(14��) �ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ��ͼ���£�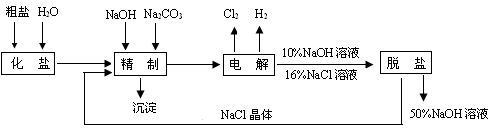
������ͼ�����������գ�
�ڵ������У����Դ���������ĵ缫����������Ӧ�ķ���ʽΪ�� ��
���Դ���������ĵ缫������ҺpH ��(����䡱������С��)
��2�����������SO42-�����ϸߣ��������ӱ��Լ���ȥSO42-�����Լ�����ѡ ��
a��Ba(OH)2 b��Ba(NO3)2 c��BaCl2
��3��Ϊ��Ч��ȥCa2����Mg2����SO42-�������Լ��ĺ���˳��Ϊ (ѡa��b��c����ѡ�۷�)��
a���ȼ�NaOH�����Na2CO3���ټӱ��Լ���
b���ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
c���ȼӱ��Լ������NaOH���ټ�Na2CO3
��4�����������ӽ���Ĥ�����ʳ��ˮʱ�����۷ָ�Ϊ������������������ֹCl2��NaOH��Ӧ��������Ĥ������ʳ��ˮʱ��Cl2��NaOH��ַ�Ӧ���������ս���NaClO��H2����Ӧ�Ļ�ѧ��Ӧ����ʽΪ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ҫ�İ뵼����ϣ��������ִ����ӹ�ҵ�Ļ������ش��������⣺
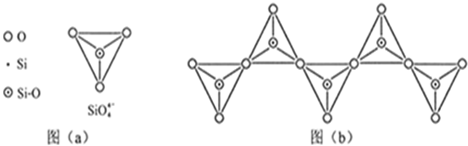
��1����̬Siԭ���У�����ռ�ݵ�����ܲ���� �����ܲ���е�ԭ�ӹ����Ϊ ��������Ϊ ��
��2������Ҫ�Թ����Ρ� �Ȼ��������ʽ�����ڵؿ��С�
��3�����ʹ��������ʯ�ṹ���Ƶľ��壬����ԭ����ԭ��֮���� ���ϣ��侧���й���8��ԭ�ӣ�����������λ�ù��� ��ԭ�ӡ�
��4�����ʹ��ͨ������(SiH4)�ֽⷴӦ���Ʊ�����ҵ�ϲ���Mg2Si��NH4CI��Һ�������з�Ӧ�Ƶ�SiH4���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��̼����йػ�ѧ������������ʾ����Ҫ�����ͽ��������й���ʵ��
| ��ѧ�� | C-C | C-H | C-O | Si-Si | Si-H | Si-O |
| ���ܣ�KJ/mol�� | 356 | 413 | 336 | 226 | 318 | 452 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijУ��ѧ�о���ѧϰС�飬��ѧϰ������ұ���Ժ��һ����̼��ԭ�����������ʵ��dz�����Ȥ�����Dz����й����Ϻ��֣�һ����̼���Ʊ������ü����Ũ���Ṳ�ȵ�60��80 �淢����ˮ��Ӧ��ȡ��
HCOOH CO����H2O
CO����H2O
��������¸�ͼ��������װ��һ����Ժ�����ʵ��װ��ͼ(ijЩװ�ÿ��ظ�ʹ��)��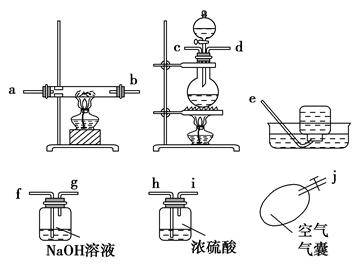
�ش��������⣺
(1)����ʵ��װ�õ�����˳����(дСд��ĸ) ��
(2)�ڷ�Ӧʱһ��Ҫ��ͨһ��һ����̼���壬Ȼ���ٵ�ȼ�����������ľƾ��ƣ�ԭ���� ��
| A����Ϊһ�㷴Ӧ�����ҽ��� |
| B���ų���ϵ�ڵĿ�����ʹ��Ӧ���̸���ȫ |
| C��������Ũ���ᷴӦ���Բ���������CO |
| D���˷�Ӧ����ʱ�䳤�����ڲ���CO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����仯ѧ�����ս����̿�(��Ҫ�ɷ�MnO2)��Ũ�����ϼ��ȣ��������������Ƶ���������д���÷�Ӧ�����ӷ���ʽ ��
��2�����ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ�� Ư���dz��õ�����������ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]��ȡƯ�ۣ���ѧ��Ӧ����ʽΪ ��
��3������ͼ������ֱ�߷ֱ��ʾ�ơ�ͭ��������������Cl2��Ӧʱ�����Ľ���������(����)�뷴Ӧ������������(����)�Ĺ�ϵ�����д�������Cl2��Ӧ��ֱ���� ����������ʾ���ĵ��������������b��ʾ�������ֽ����е� ��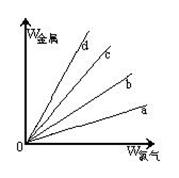
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ʯ��SiC������������ĥ����ʴ���ԣ�Ӧ�ù㷺��
(1)̼�������Ԫ��Q�ĵ��ʻ��Ͻ����������ֳ�����̬���������һ�ֻ�����RΪ�Ǽ��Է��ӡ�̼Ԫ�������ڱ��е�λ����____________��Q��____________��R�ĵ���ʽΪ________��
(2)һ�������£�Na��ԭCCl4���Ʊ����ʯ����Ӧ������ȴ�����º������е�CCl4��ʵ���������Ϊ________����ȥ�ֲ�Ʒ�������Ƶ��Լ�Ϊ________��
(3)̼��ԭSiO2��SiC����ֲ�Ʒ������ΪSi��SiO2���ֽ�20.0 g SiC�ֲ�Ʒ���뵽������NaOH��Һ�г�ַ�Ӧ���ռ���0.1 mol���������˵�SiC����11.4 g����Һϡ�͵�1 L���������������ӷ���ʽΪ__________________________________�������ε����ʵ���Ũ��Ϊ_________��
(4)����������ȷ����________(�����)��
��Na��ԭCCl4�ķ�Ӧ��Cl2��H2O�ķ�Ӧ�����û���Ӧ
��ˮ�����ɱ��ۻ�ʱ�˷����Ӽ���������������ͬ
��Na2SiO3��Һ��SO3�ķ�Ӧ�������ƶ�Si��S�ķǽ�����ǿ��
���ơ�﮷ֱ��ڿ�����ȼ�գ����ɵ�������������������Ŀ�Ⱦ�Ϊ1��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧС�����Na2SO3������ʵ��̽����
�ڰ�ɫ��ΰ��a��b��c���������е���Na2SO3��Һ���ٷֱ�μ���ͼ��ʾ���Լ���
ʵ���������±���
| ��� | ʵ������ |
| a | ��ˮ��ɫ |
| b | ��������ɫ���� |
| c | �����̪��Һ��죬�ټ���BaCl2��Һ����������Һ�ɫ��ȥ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com