
ij��ѧ��ȤС����ѧϰ����Ļ������ijЩ���ʡ��У�����������ʵ�飺
��ʵ��һ��̽��SO2�����ʣ�
����ͼ��ʾװ�ý���ʵ�顣

��ش��������⣺
��1��װ��A��ʢ���������Ƶ����������� �����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ������У�װ��B��C�з���������ֱ��� �� ����Щ����ֱ�˵��SO2���е������� �� ��װ��B�з�����Ӧ�����ӷ���ʽΪ ��
��3��װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������
______________________________________________��
��4����װ����һ����ȱ�ݣ���ָ��_______________________�����ڿ�ͼ�ڻ�������װ�ã�ע��ҩƷ����

��ʵ�������֤����п��Ũ���ᷴӦ����������ɷ��Ƕ������������������ͼװ�ý���ʵ�飨п��Ũ���Ṳ��ʱ����������ΪX���Ҹ�װ����ȥ�����Իش�

��5��A�м�����Լ�������____________��������_______________��
B�м�����Լ�������____________��������_______________��
E�м�����Լ�������____________��������_______________��
��6������֤������X�к���������ʵ�������ǣ�C�У�____________��D�У�__________��
��20�֣���ʵ��һ��(1)������ƿ Na2SO3+H2SO4(Ũ)��Na2SO4+SO2��
(2)��Һ���Ϻ�ɫ��Ϊ��ɫ����ɫ��Һ���ֻ�ɫ���� ��ԭ�Ժ�������
5SO2+2MnO4-+2H2O��5SO42�� +2Mn2++4H+
(3)Ʒ����Һ��ɫ�رշ�Һ©����������ȼ�Ĵ��ƾ��Ƽ��ȣ���Һ�ָ���ɫ
(4)ȱ��β������װ�� 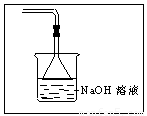
��ʵ�����
��5��Ʒ����Һ�� ����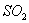 ��
��
Ũ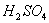 �� ����ˮ������
�� ����ˮ������
��ʯ�ң� ���Ͽ�����ˮ��������D��
��6��C�к�ɫ��ĩ��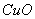 ����ɺ�ɫ
����ɺ�ɫ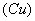 ��D�а�ɫ��ĩ�����ɫ��
��D�а�ɫ��ĩ�����ɫ��
��������(4 ��)��1��0.04 (2)26.7
�����������ʵ��һ����1�����������Ľṹ��֪��װ��A��ʢ���������Ƶ�����������������ƿ�����з�����Ӧ�Ļ�ѧ����ʽ��Na2SO3+H2SO4(Ũ)��Na2SO4+SO2����
��2��SO2���л�ԭ�ԣ��ܱ����Ը��������Һ����������B����������Һ���Ϻ�ɫ��Ϊ��ɫ����Ӧ�����ӷ���ʽ��5SO2+2MnO4-+2H2O��5SO42�� +2Mn2++4H+��SO2Ҳ���������ԣ��ܰ������������ɵ���S����������C����������ɫ��Һ���ֻ�ɫ���ǡ�
��3��SO2��Ư���Dz��ȶ��ģ��������ܻӷ�ԭ������ɫ������Ҫ̽��SO2��Ʒ�����õĿ����ԣ�ʵ�������������Ʒ����Һ��ɫ�رշ�Һ©����������ȼ�Ĵ��ƾ��Ƽ��ȣ���Һ�ָ���ɫ��
��4��SO2�Ǵ�����Ⱦ�ȱ��β������װ�á�
��ʵ�������5������SO2���Լ���Ʒ����Һ����A�е��Լ���Ʒ����Һ����������SO2�����������к���ˮ����������������ļ��飬���B�е��Լ�Ũ���ᣬ��������ˮ���������������Ҳ����ˮ������ʣ��E��Ӧ��ʢ�ż�ʯ�ң��������Ͽ�����ˮ��������D�С�
��6��������ԭ����ͭ����ͭ��ˮ���������Կ���֤������X�к���������ʵ�������ǣ�C�к�ɫ��ĩ��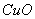 ����ɺ�ɫ
����ɺ�ɫ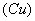 ��D�а�ɫ��ĩ�����ɫ��
��D�а�ɫ��ĩ�����ɫ��
���㣺����������ʶ�𣬻���ʵ�������SO2���Ʊ������顢�����Լ�Ư����̽��
�����������Ǹ߿��еij������ͣ��Ѷȴ��ۺ���ǿ����ѧ����Ҫ��ߡ�������ע�ضԻ���֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ���淶�Ͻ���ʵ����������Լ���������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ����������������������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�


 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣�ij��ѧ��ȤС����ѧϰ�˵���ʵ��й�֪ʶ����ʦ�����һ���������ˮ��Һ����������ǿ����Ӱ�����ء��Ŀ��⡣�ø�С������ɿ�����о�����֪HCl��ˮ������ȫ���롣
��1����С��ͬѧ�������ܶ�Ϊ1.049 g��cm-3�����ᣨCH3COOH������������Ϊ36.5%���ܶ�Ϊ1.18 g��cm-3��Ũ����ֱ�����1mol/L CH3COOH��Һ��1 mol/L HCl��Һ��500 mL��������Ӧ����ȡ�����Ũ���������ֱ�Ϊ �� ��
��2��ijͬѧ������500 mL 1 mol/L��CH3COOH��Һʱ��������ƿ��ת����Һʱ������ͼ��ʾ��ͼ�еĴ����� ��
��3��������������ͼ��ʾװ�ò�1 mol/L��CH3COOH��Һ��1mol/L HCl��Һ�ĵ�����������ͨ��Դ������HCl��Һ�����ĵ��ݽ�������ͬѧ��Ϊ������������ʣ��� ���ͬ�⡱��ͬ�⡱�����Ĺ۵㣬ͨ����������õ��Ľ�����
��4��������������װ�ò����ʵ���Ũ����ͬ��CuSO4��Һ��NaOH��Һ�ĵ�����������ͨ��Դ������CuSO4��Һ�����ĵ��ݽ�������ͬѧ��ΪNaOH��������ʣ��� ���ͬ�⡱��ͬ�⡱�����Ĺ۵㣬ͨ����������õ��Ľ����� ��
��5��ͨ������̽��ʵ�飬���ܵõ��Ľ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣�ij��ѧ��ȤС����ѧϰ�˵���ʵ��й�֪ʶ����ʦ�����һ���������ˮ��Һ����������ǿ����Ӱ�����ء��Ŀ��⡣�ø�С������ɿ�����о�����֪HCl��ˮ������ȫ���롣
��1����С��ͬѧ�������ܶ�Ϊ1.049 g��cm-3�����ᣨCH3COOH������������Ϊ36.5%���ܶ�Ϊ1.18 g��cm-3��Ũ����ֱ�����1mol/L CH3COOH��Һ��1 mol/L HCl��Һ��500 mL��������Ӧ����ȡ�����Ũ���������ֱ�Ϊ �� ��
��2��ijͬѧ������500 mL 1 mol/L��CH3COOH��Һʱ��������ƿ��ת����Һʱ������ͼ��ʾ��ͼ�еĴ����� ��
��3��������������ͼ��ʾװ�ò�1 mol/L��CH3COOH��Һ��1mol/L HCl��Һ�ĵ�����������ͨ��Դ������HCl��Һ�����ĵ��ݽ�������ͬѧ��Ϊ������������ʣ��� ���ͬ�⡱��ͬ�⡱�����Ĺ۵㣬ͨ����������õ��Ľ�����
��4��������������װ�ò����ʵ���Ũ����ͬ��CuSO4��Һ��NaOH��Һ�ĵ�����������ͨ��Դ������CuSO4��Һ�����ĵ��ݽ�������ͬѧ��ΪNaOH��������ʣ��� ���ͬ�⡱��ͬ�⡱�����Ĺ۵㣬ͨ����������õ��Ľ����� ��
��5��ͨ������̽��ʵ�飬���ܵõ��Ľ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ���ݵڶ���ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��9�֣�ij��ѧ��ȤС����ѧϰ�˵���ʵ��й�֪ʶ����ʦ�����һ���������ˮ��Һ����������ǿ����Ӱ�����ء��Ŀ��⡣�ø�С������ɿ�����о�����֪HCl��ˮ������ȫ���롣
��1����С��ͬѧ�������ܶ�Ϊ1.049 g��cm-3�����ᣨCH3COOH������������Ϊ36.5%���ܶ�Ϊ1.18 g��cm-3��Ũ����ֱ�����1 mol/L CH3COOH��Һ��1 mol/L HCl��Һ��500 mL��������Ӧ����ȡ�����Ũ���������ֱ�Ϊ �� ��
��2��ijͬѧ������500 mL 1 mol/L��CH3COOH��Һʱ��������ƿ��ת����Һʱ������ͼ��ʾ��ͼ�еĴ����� ��
��3��������������ͼ��ʾװ�ò�1 mol/L��CH3COOH��Һ��1 mol/L HCl��Һ�ĵ�����������ͨ��Դ������HCl��Һ�����ĵ��ݽ�������ͬѧ��Ϊ������������ʣ��� ���ͬ�⡱��ͬ�⡱�����Ĺ۵㣬ͨ����������õ��Ľ����� 
��4��������������װ�ò����ʵ���Ũ����ͬ��CuSO4��Һ��NaOH��Һ�ĵ�����������ͨ��Դ������CuSO4��Һ�����ĵ��ݽ�������ͬѧ��ΪNaOH��������ʣ��� ���ͬ�⡱��ͬ�⡱�����Ĺ۵㣬ͨ����������õ��Ľ����� ��
��5��ͨ������̽��ʵ�飬���ܵõ��Ľ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��9�֣�ij��ѧ��ȤС����ѧϰ�˵���ʵ��й�֪ʶ����ʦ�����һ���������ˮ��Һ����������ǿ����Ӱ�����ء��Ŀ��⡣�ø�С������ɿ�����о�����֪HCl��ˮ������ȫ���롣
��1����С��ͬѧ�������ܶ�Ϊ1.049 g��cm-3�����ᣨCH3COOH������������Ϊ36.5%���ܶ�Ϊ1.18 g��cm-3��Ũ����ֱ�����1 mol/L CH3COOH��Һ��1 mol/L HCl��Һ��500 mL��������Ӧ����ȡ�����Ũ���������ֱ�Ϊ �� ��
��2��ijͬѧ������500 mL 1 mol/L��CH3COOH��Һʱ��������ƿ��ת����Һʱ������ͼ��ʾ��ͼ�еĴ����� ��

��3��������������ͼ��ʾװ�ò�1 mol/L��CH3COOH��Һ��1 mol/L HCl��Һ�ĵ�����������ͨ��Դ������HCl��Һ�����ĵ��ݽ�������ͬѧ��Ϊ������������ʣ��� ���ͬ�⡱��ͬ�⡱�����Ĺ۵㣬ͨ����������õ��Ľ�����

��4��������������װ�ò����ʵ���Ũ����ͬ��CuSO4��Һ��NaOH��Һ�ĵ�����������ͨ��Դ������CuSO4��Һ�����ĵ��ݽ�������ͬѧ��ΪNaOH��������ʣ��� ���ͬ�⡱��ͬ�⡱�����Ĺ۵㣬ͨ����������õ��Ľ����� ��
��5��ͨ������̽��ʵ�飬���ܵõ��Ľ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com