
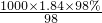 mol/L=18.4mol/L£¬øł¾ŻĻ”ŹĶ¶ØĀÉ£¬Ļ”ŹĶĒ°ŗóČÜÖŹµÄĪļÖŹµÄĮæ²»±ä£¬Ą“¼ĘĖćÅØĮņĖįµÄĢå»ż£¬ÉčÅØĮņĖįµÄĢå»żĪŖxmL£¬ĖłŅŌxmL”Į18.4mol/L=250mL”Į0.5mol/L£¬½āµĆ£ŗx”Ö6.8£¬ĖłŅŌÓ¦ĮæČ”µÄÅØĮņĖįĢå»żŹĒ6.8mL£¬¹ŹŃ”10mLĮæĶ²£®
mol/L=18.4mol/L£¬øł¾ŻĻ”ŹĶ¶ØĀÉ£¬Ļ”ŹĶĒ°ŗóČÜÖŹµÄĪļÖŹµÄĮæ²»±ä£¬Ą“¼ĘĖćÅØĮņĖįµÄĢå»ż£¬ÉčÅØĮņĖįµÄĢå»żĪŖxmL£¬ĖłŅŌxmL”Į18.4mol/L=250mL”Į0.5mol/L£¬½āµĆ£ŗx”Ö6.8£¬ĖłŅŌÓ¦ĮæČ”µÄÅØĮņĖįĢå»żŹĒ6.8mL£¬¹ŹŃ”10mLĮæĶ²£® £¬ŌŁøł¾ŻČÜŅŗĻ”ŹĶĒ°ŗóĪļÖŹµÄĮæ²»±ä¼ĘĖćĖłŠčÅØĮņĖįµÄĢå»ż£¬øł¾ŻÅØĮņĖįµÄĢå»żŃ”ŌńĮæĶ²µÄ¹ęøń£®
£¬ŌŁøł¾ŻČÜŅŗĻ”ŹĶĒ°ŗóĪļÖŹµÄĮæ²»±ä¼ĘĖćĖłŠčÅØĮņĖįµÄĢå»ż£¬øł¾ŻÅØĮņĖįµÄĢå»żŃ”ŌńĮæĶ²µÄ¹ęøń£® ÅŠ¶Ļ£®
ÅŠ¶Ļ£® Ąķ½āÅäÖĘŌĄķ£¬×¢ŅāÅØĮņĖįµÄĻ”ŹĶ²Ł×÷£®
Ąķ½āÅäÖĘŌĄķ£¬×¢ŅāÅØĮņĖįµÄĻ”ŹĶ²Ł×÷£® 


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŠĀŠŽøĵĔ¶»ś¶Æ³µ¼ŻŹ»Ö¤ÉźĮģŗĶŹ¹ÓĆ¹ę¶Ø”·ÓŚ2010Äź4ŌĀ1ČÕĘšŹµŹ©£¬ŠĀ¹ę¹ę¶Ø¾Ę¼ŻŅ»“ĪæŪ12·Ö£¬³ö“ĖÖŲČÖĪĄķ¾Ę¼ŻŹĒŅņĪŖ¾Ęŗó¼Ż³µŹĒŅż·¢½»ĶØŹĀ¹ŹµÄÖŲŅŖŌŅņ£®ČēĶ¼ĪŖ½»¾Æ¶Ō¼ŻŹ»Ō±ŹĒ·ńŅū¾Ę½ųŠŠ¼ģ²ā£®ĘäŌĄķČēĻĀ£ŗ
ŠĀŠŽøĵĔ¶»ś¶Æ³µ¼ŻŹ»Ö¤ÉźĮģŗĶŹ¹ÓĆ¹ę¶Ø”·ÓŚ2010Äź4ŌĀ1ČÕĘšŹµŹ©£¬ŠĀ¹ę¹ę¶Ø¾Ę¼ŻŅ»“ĪæŪ12·Ö£¬³ö“ĖÖŲČÖĪĄķ¾Ę¼ŻŹĒŅņĪŖ¾Ęŗó¼Ż³µŹĒŅż·¢½»ĶØŹĀ¹ŹµÄÖŲŅŖŌŅņ£®ČēĶ¼ĪŖ½»¾Æ¶Ō¼ŻŹ»Ō±ŹĒ·ńŅū¾Ę½ųŠŠ¼ģ²ā£®ĘäŌĄķČēĻĀ£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com