

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
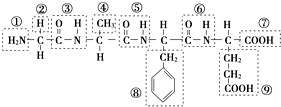
 £Ø1£©Ä³»ÆŗĻĪļ½į¹¹¼ņŹ½ČēĶ¼£¬Ēėøł¾ŻĖłŹ¾µÄ»ÆŗĻĪļ»Ų“šĪŹĢā£ŗ
£Ø1£©Ä³»ÆŗĻĪļ½į¹¹¼ņŹ½ČēĶ¼£¬Ēėøł¾ŻĖłŹ¾µÄ»ÆŗĻĪļ»Ų“šĪŹĢā£ŗ
| Ļ”ĮņĖį |
| Ļ”ĮņĖį |
| Ļ”ĮņĖį |
| Ļ”ĮņĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
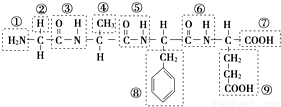
£Ø12·Ö£©¢ń ij»ÆŗĻĪļ½į¹¹¼ņŹ½ČēĶ¼£¬Ēėøł¾ŻĖłŹ¾µÄ»ÆŗĻĪļ»Ų“šĪŹĢā£ŗ
£Ø1£©øĆ»ÆŗĻĪļÖŠ£¬¹ŁÄÜĶÅ¢ŁµÄĆū³ĘŹĒ________£»¹ŁÄÜĶŢߵÄĆū³ĘŹĒ________£»øĆ»ÆŗĻĪļŹĒÓÉ________øö°±»łĖį·Ö×ÓĶŃĖ®ŠĪ³ÉµÄ£»
£Ø2£©Š“³öøĆ»ÆŗĻĪļĖ®½āÉś³ÉµÄČĪŅāŅ»ÖÖ°±»łĖįÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_________________
¢ņ Ź³Ę·°²Č«¹ŲĻµ×ŹśĆńÉś¼Ę£¬Ó°ĻģŹ³Ę·°²Č«µÄŅņĖŲŗܶą”£
£Ø3£©¾ŪĘ«¶žĀČŅŅĻ©(??)¾ßÓŠ³¬Ēæ×čøōŠŌÄÜ£¬æÉ×÷ĪŖ±£ĻŹŹ³Ę·µÄ°ü×°²ÄĮĻ”£ĖüŹĒÓÉ________(Š“½į¹¹¼ņŹ½)·¢Éś¼Ó¾Ū·“Ӧɜ³ÉµÄ”£
(4)ĮÓÖŹÖ²ĪļÓĶÖŠµÄŃĒÓĶĖį[CH3(CH2)4”ŖCH===CH”ŖCH2”ŖCH===CH”Ŗ(CH2)7COOH]ŗ¬ĮæŗܵĶ”£ĻĀĮŠ¹ŲÓŚŃĒÓĶĖįµÄĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ________”££ØĢīŠņŗÅ£©
A£®·Ö×ÓŹ½ĪŖC18H34O2
B£®Ņ»¶ØĢõ¼žĻĀÄÜÓėøŹÓĶ(±ūČż“¼)·¢Éśõ„»Æ·“Ó¦
C£®ÄÜŗĶNaOHČÜŅŗ·“Ó¦
D£®ÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ«
(5)¼Ł¾ĘÖŠ¼×“¼(CH3OH)ŗ¬Į泬±ź£¬ĒėŠ“³öNaŗĶ¼×“¼·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ______________________”£
£Ø6£©µķ·Ū×īÖÕµÄĖ®½ā²śĪļŹĒĘĻĢŃĢĒ”£ĒėÉč¼ĘŹµŃéÖ¤Ć÷µķ·ŪŅŃ¾Č«²æĖ®½ā£¬Š“³ö²Ł×÷”¢ĻÖĻóŗĶ½įĀŪ£ŗ______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗÓ±±Ź”ŗāĖ®ÖŠŃ§ø߶žÉĻѧʌĖĵ÷æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©¢ńij»ÆŗĻĪļ½į¹¹¼ņŹ½ČēĶ¼£¬Ēėøł¾ŻĖłŹ¾µÄ»ÆŗĻĪļ»Ų“šĪŹĢā£ŗ
£Ø1£©øĆ»ÆŗĻĪļÖŠ£¬¹ŁÄÜĶÅ¢ŁµÄĆū³ĘŹĒ________£»¹ŁÄÜĶŢߵÄĆū³ĘŹĒ________£»øĆ»ÆŗĻĪļŹĒÓÉ________øö°±»łĖį·Ö×ÓĶŃĖ®ŠĪ³ÉµÄ£»
£Ø2£©Š“³öøĆ»ÆŗĻĪļĖ®½āÉś³ÉµÄČĪŅāŅ»ÖÖ°±»łĖįÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_________________
¢ņ Ź³Ę·°²Č«¹ŲĻµ×ŹśĆńÉś¼Ę£¬Ó°ĻģŹ³Ę·°²Č«µÄŅņĖŲŗܶą”£
£Ø3£©¾ŪĘ«¶žĀČŅŅĻ©(? ?)¾ßÓŠ³¬Ēæ×čøōŠŌÄÜ£¬æÉ×÷ĪŖ±£ĻŹŹ³Ę·µÄ°ü×°²ÄĮĻ”£ĖüŹĒÓÉ________(Š“½į¹¹¼ņŹ½)·¢Éś¼Ó¾Ū·“Ӧɜ³ÉµÄ”£
?)¾ßÓŠ³¬Ēæ×čøōŠŌÄÜ£¬æÉ×÷ĪŖ±£ĻŹŹ³Ę·µÄ°ü×°²ÄĮĻ”£ĖüŹĒÓÉ________(Š“½į¹¹¼ņŹ½)·¢Éś¼Ó¾Ū·“Ӧɜ³ÉµÄ”£
(4)ĮÓÖŹÖ²ĪļÓĶÖŠµÄŃĒÓĶĖį[CH3(CH2)4”ŖCH===CH”ŖCH2”ŖCH===CH”Ŗ(CH2)7COOH]ŗ¬ĮæŗܵĶ”£ĻĀĮŠ¹ŲÓŚŃĒÓĶĖįµÄĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ________”££ØĢīŠņŗÅ£©
| A£®·Ö×ÓŹ½ĪŖC18H34O2 |
| B£®Ņ»¶ØĢõ¼žĻĀÄÜÓėøŹÓĶ(±ūČż“¼)·¢Éśõ„»Æ·“Ó¦ |
| C£®ÄÜŗĶNaOHČÜŅŗ·“Ó¦ |
| D£®ÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÕć½Ź”įéÖŻŅ»ÖŠø߶ž£ØÉĻ£©ĘŚÖŠ»ÆѧŹŌ¾ķ£ØĄķ£©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
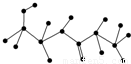
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com