
Š“³öĻĀĮŠÓŠ»śĪļµÄ½į¹¹¼ņŹ½£¬ČōÓŠĪ„·“ĻµĶ³ĆüĆūÕßĒėÓčŅŌ¾ĄÕż”£
(1)3,5¶ž¼×»ł¼ŗĶé
____________________£¬ÕżČ·Ćū³Ę£ŗ____________£»
(2)3,3,4,4Ėļ׻ł2ŅŅ»łĪģĶé
____________________£¬ÕżČ·Ćū³Ę£ŗ____________£»
(3)4,4,5,5Ėļ׻ł3±ū»ł¼ŗĶé
____________________£¬ÕżČ·Ćū³Ę£ŗ____________£»
(4)2,3,4,5Ėļ׻ł3ŅŅ»ł5±ū»łøżĶé
____________________£¬ÕżČ·Ćū³Ę£ŗ____________”£
 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×éŅŗĢå»ģŗĻĪļÖŠÓŠĮ½Öֳɷ֣¬æÉŅŌÓĆ·ÖŅŗĀ©¶··ÖĄėµÄŹĒ(””””)
A£®¼×±½ŗĶÓĶÖ¬ B£®ŅŅĖįŗĶŅŅĖįŅŅõ„
C£®¶¹ÓĶŗĶĖ® D£®¶¹ÓĶŗĶĖÄĀČ»ÆĢ¼
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”¢BĮ½ÖÖÓŠ»śĪļ£¬·Ö×ÓŹ½¶¼ŹĒC9H11O2N”£
(1)»ÆŗĻĪļAŹĒĢģČ»µ°°×ÖŹµÄĖ®½ā²śĪļ£¬¹āĘײā¶ØĻŌŹ¾£¬·Ö×Ó½į¹¹ÖŠ²»“ęŌŚ”ŖCH3£¬»ÆŗĻĪļAµÄ½į¹¹¼ņŹ½ĪŖ
________________________________________________________________________ӣ
(2)»ÆŗĻĪļBŹĒijÖÖ·Ö×ÓŹ½ĪŖC9H12µÄ·¼ĻćĢžŅ»Ļõ»ÆŗóµÄĪØŅ»²śĪļ(Ļõ»łĮ¬ŌŚ±½»·ÉĻ)”£»ÆŗĻĪļBµÄ½į¹¹¼ņŹ½ĪŖ
________________________________________________________________________ӣ
(3)Į½·Ö×ÓAæÉŠĪ³Éŗ¬ÓŠĮłŌŖ»·µÄĪļÖŹC£¬ŌņCµÄ½į¹¹¼ņŹ½ĪŖ
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ£¬ŌņĻĀĮŠŠšŹöÕżČ·µÄŹĒ(””””)
A£®³£ĪĀ³£Ń¹ĻĀ£¬46 gÓŠ»śĪļC2H6OÖŠŗ¬ÓŠ¼«ŠŌ¼üµÄŹżÄæŅ»¶ØĪŖ7NA
B£®±ź×¼×“æöĻĀ£¬22.4 LĖÄĀČ»ÆĢ¼ÖŠĖłŗ¬ÓŠµÄ¹²¼Ū¼üŹżÄæĪŖ4NA
C£®±ź×¼×“æöĻĀ£¬5.6 L NOŗĶ5.6 L O2×é³ÉµÄ»ģŗĻĘųĢåÖŠĖłŗ¬Ō×ÓŹżĪŖNA
D£®³£ĪĀ³£Ń¹ĻĀ£¬33.6 LĀČĘųÓė56 gĢś³ä·Ö·“Ó¦£¬×ŖŅʵĵē×ÓŹżĪŖ3NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÖʵƵĵŖ»ÆĀĮ(AlN)²śĘ·ÖŠ³£ŗ¬ÓŠÉŁĮæAl4C3”¢Al2O3”¢CµČŌÓÖŹ”£Ä³Ķ¬Ń§Éč¼ĘĮĖČēĻĀŹµŃé·Ö±š²ā¶ØµŖ»ÆĀĮ(AlN)ѳʷ֊AlNŗĶAl4C3µÄÖŹĮæ·ÖŹż(ŗöĀŌNH3ŌŚĒæ¼īŠŌČÜŅŗÖŠµÄČܽā)”£
£Ø1£©ŹµŃéŌĄķ
¢ŁAl4C3ÓėĮņĖį·“Ó¦æÉÉś³ÉCH4£»
¢ŚAlNČÜÓŚĒæĖį²śÉśļ§ŃĪ£¬ČÜÓŚĒæ¼īÉś³É°±Ęų£¬ĒėŠ“³öAlNÓėNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©ŹµŃé×°ÖĆ(ČēĶ¼ĖłŹ¾)

£Ø3£©ŹµŃé¹ż³Ģ
¢ŁĮ¬½ÓŹµŃé×°ÖĆ£¬¼ģŃé×°ÖƵÄĘųĆÜŠŌ”£³ĘµĆD×°ÖƵÄÖŹĮæĪŖyg£¬µĪ¶Ø¹ÜµÄ¶ĮŹżĪŖamL”£
¢Ś³ĘČ”xg AlNѳʷÖĆӌ׶ŠĪĘæÖŠ£»ČūŗĆ½ŗČū£¬¹Ų±Õ»īČū £¬“ņæŖ»īČū £¬Ķعż·ÖŅŗĀ©¶·¼ÓČėĻ”ĮņĖį£¬ÓėÉÕĘæÄŚĪļÖŹ³ä·Ö·“Ó¦”£
¢Ū“ż·“Ó¦½ųŠŠĶźČ«ŗ󣬹Ų±Õ»īČū £¬“ņæŖ»īČū £¬Ķعż·ÖŅŗĀ©¶·¼ÓČė¹żĮæ
(Ģī»ÆѧŹ½)£¬ÓėÉÕĘæÄŚĪļÖŹ³ä·Ö·“Ó¦”£
¢Ü (ĢīČėøĆ²½Ó¦½ųŠŠµÄ²Ł×÷)”£
¢Ż¼ĒĀ¼µĪ¶Ø¹ÜµÄ¶ĮŹżĪŖbmL£¬³ĘµĆD×°ÖƵÄÖŹĮæĪŖzg”£
£Ø4£©Źż¾Ż·ÖĪö
¢ŁAlNµÄÖŹĮæ·ÖŹżĪŖ ”£
¢ŚČō¶ĮČ”µĪ¶Ø¹ÜÖŠĘųĢåµÄĢå»żŹ±£¬ŅŗĆę×óøßÓŅµĶ£¬ŌņĖł²āĘųĢåµÄĢå»ż (Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±)”£
¢ŪAl4C3µÄÖŹĮæ·ÖŹżĪŖ (øĆŹµŃéĢõ¼žĻĀµÄĘųĢåĦ¶ūĢå»żĪŖVm)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
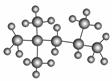
ŠĮĶéÖµ³£ÓĆĄ“±ķŹ¾ĘūÓĶµÄÖŹĮ棬ĘūÓĶÖŠŅģŠĮĶéµÄ±¬Õš³Ģ¶Č×īŠ”£¬Ņ»°ć½«ĘäŠĮĶéÖµ±ź¶ØĪŖ100”£ČēĶ¼ŹĒŅģŠĮĶé·Ö×ÓµÄĒņ¹÷Ä£ŠĶ£¬ŌņŅģŠĮĶéµÄĻµĶ³ĆüĆūĪŖ(””””)
A£®1,1,3,3Ėļ׻ł¶”Ķé B£®2,2,4Čż¼×»łĪģĶé
C£®2,4,4Čż¼×»łĪģĶé D£®2,3,4Čż¼×»łĪģĶé
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā”£
(1) 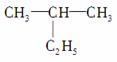 ĆüĆūĪŖ”°2ŅŅ»ł±ūĶé”±£¬“ķĪóŌŅņŹĒ____________________£»½«
ĆüĆūĪŖ”°2ŅŅ»ł±ūĶé”±£¬“ķĪóŌŅņŹĒ____________________£»½«
ĘäĆüĆūĪŖ”°3¼×»ł¶”Ķé”±£¬“ķĪóŌŅņŹĒ__________________”£ÕżČ·µÄĆüĆūĪŖ________”£
(2)  µÄĆū³ĘŹĒ________________”£
µÄĆū³ĘŹĒ________________”£
(3)2,6¶ž¼×»ł4ŅŅ»łŠĮĶéµÄ½į¹¹¼ņŹ½ŹĒ______________________£¬1 moløĆĶéĢžĶźČ«Č¼ÉÕŠčĻūŗÄŃõĘų______mol”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤Ņµ·ĻĖ®ÖŠ³£ŗ¬ÓŠŅ»¶ØĮæµÄCr2O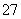 ŗĶCrO
ŗĶCrO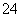 £¬ĖüĆĒ»į¶ŌÉśĢ¬ĻµĶ³Ōģ³ÉŗÜ“óµÄĖšŗ¦£¬ĘäÖŠ»¹Ō³Įµķ·ØŹĒ³£ÓƵÄŅ»ÖÖ“¦Ąķ·½·Ø”£Į÷³ĢČēĻĀ£ŗ
£¬ĖüĆĒ»į¶ŌÉśĢ¬ĻµĶ³Ōģ³ÉŗÜ“óµÄĖšŗ¦£¬ĘäÖŠ»¹Ō³Įµķ·ØŹĒ³£ÓƵÄŅ»ÖÖ“¦Ąķ·½·Ø”£Į÷³ĢČēĻĀ£ŗ
CrO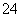
 Cr2O
Cr2O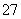
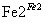 Cr3£«
Cr3£« Cr(OH)3”ż
Cr(OH)3”ż
ĘäÖŠµŚ¢Ł²½ÖŠ“ęŌŚĘ½ŗā£ŗ2CrO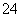 (»ĘÉ«)£«2H£«
(»ĘÉ«)£«2H£« Cr2O
Cr2O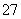 (³ČÉ«)£«H2O”£ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ
(³ČÉ«)£«H2O”£ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ
A”¢µŚ¢Ł²½µ±2v(Cr2O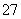 )£½v(CrO
)£½v(CrO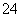 )Ź±£¬“ļµ½ĮĖĘ½ŗāדĢ¬
)Ź±£¬“ļµ½ĮĖĘ½ŗāדĢ¬
B”¢¶ŌÓŚÉĻŹöĘ½ŗā£¬¼ÓČėŹŹĮæĻ”ĮņĖįŗó£¬ČÜŅŗŃÕÉ«±ä»ĘÉ«£¬ŌņÓŠĄūÓŚCrO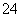 µÄÉś³É
µÄÉś³É
C”¢³£ĪĀĻĀ£¬Cr£ØOH£©3µÄČܶȻżKsp=10-32£¬ŅŖŹ¹c£ØCr3+£©½µÖĮ10-5mol/L£¬ČÜŅŗµÄpHÓ¦µ÷ÖĮ9
D”¢µŚ¢Ś²½ÖŠ£¬»¹Ō0.1 mol Cr2O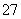 ŠčŅŖ91.2 g FeSO4
ŠčŅŖ91.2 g FeSO4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”¢B”¢C”¢D”¢E¾łŹĒ¶ĢÖÜĘŚŌŖĖŲ£¬AŗĶBĶ¬ÖÜĘŚ£¬AŗĶCĶ¬×壬AŌ×Ó×īĶā²ćµē×ÓŹżŹĒÄŚ²ćµē×ÓŹżµÄ¶ž±¶£¬BŌŖĖŲ×åŠņŹżŹĒÖÜĘŚŹżµÄČż±¶£¬BµÄŅõĄė×ÓÓėDµÄŃōĄė×Óµē×Ó²ć½į¹¹ĻąĶ¬£¬DµÄµ„ÖŹÓėBµÄµ„ÖŹŌŚ²»Ķ¬Ģõ¼žĻĀ·“Ó¦£¬æÉÉś³ÉD2B»ņD2B2£¬EŹĒĖłŌŚÖÜĘŚÖŠŌ×Ó°ė¾¶×īŠ”µÄŌŖĖŲ£¬Ēė»Ų“š”£
£Ø1£©CŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ_______.
£Ø2£©D2BµÄµē×ÓŹ½ŹĒ___________£»AB2µÄ½į¹¹Ź½ŹĒ____________.
£Ø3£©B”¢D”¢EĄė×Ó°ė¾¶µÄÓɓ󵽊”µÄĖ³ŠņĪŖ___________£»£ØÓĆĄė×Ó·ūŗÅ»Ų“š£©A”¢C”¢E×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĖįŠŌÓÉĒæµ½ČõµÄĖ³ŠņĪŖ_________.£ØÓĆ»ÆѧŹ½»Ų“š£©
£Ø4£©A”¢BĘųĢ¬Ēā»ÆĪļ·ŠµćøߵďĒ___________£»£ØÓĆ»ÆѧŹ½»Ų“š£©ŌŅņ_____________.
£Ø5£©Ė®ÖŠĆĢŗ¬Į泬±ź£¬ČŻŅ׏¹½ą¾ßŗĶŅĀĪļČ¾É«£¬Ź¹Ė®²śÉśŅŌĪŖ£¬EB2æÉŅŌÓĆĄ“³żČ„Ė®ÖŠ³¬±źµÄMn2+£¬Éś³ÉŗŚÉ«³Įµķ£¬µ±ĻūŗÄ13.50g EB2Ź±£¬¹²×ŖŅĘĮĖ1molµē×Ó£¬Ōņ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_________________.
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com