
����Ŀ����ҵ�ϳ�����������ʢװ��Ũ���ᡣΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�����
[̽��һ]
��1������ȥ�����������������(̼�ظ�)������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ����__________________��
��2������ȡ����6.0 g����15.0 mLŨ�����У����ȣ���ַ�Ӧ��õ���ҺX���ռ�������Y��
�ټ�ͬѧ��ΪX�г�Fe3������ܺ���Fe2������Ҫȷ�����е�Fe2����Ӧѡ��_________��
a��KSCN��Һ����ˮ�� b�����ۺ�KSCN��Һ
c��Ũ��ˮ d������KMnO4��Һ
����ͬѧȡ448 mL(��״��)����Yͨ��������ˮ�У��������з�Ӧ��SO2��Br2��2H2O===2HBr��H2SO4��Ȼ���������BaCl2��Һ�����ʵ�������ø������2.33 g���ɴ���֪����Y��SO2���������Ϊ________��
[̽����] ��������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ���H2��Q���塣Ϊ�����������̽��ʵ��װ��(ͼ�мг�����ʡ��)��
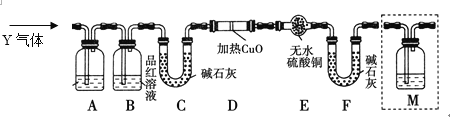
��3��װ��B��������__________________________________________________��
��4����Ϊ����Y�л�����Q��������____________________ (�û�ѧ����ʽ��ʾ)��
��5��Ϊȷ��Q�Ĵ��ڣ���Ҫ�õ�װ��M����M��װ���Լ���______________������M������________(ѡ�����)��
a��A֮ǰ b��A��B�� c��B��C�� d��C��D��
��6���������Y�к���H2��Ԥ��ʵ������Ӧ��_____________________________��
��7����ij���������CO2��SO2��H2�е�һ�ֻ������ɣ����ⶨ����������������Ϊ50%������������ɿ���Ϊ___________������ţ���
a��SO2 b��H2��SO2 c��H2��CO2 d��CO2��SO2 e��SO2��CO2��H2
���𰸡� �������汻�ۻ� d 50% ����SO2�Ƿ���� C��2H2SO4(Ũ)![]() CO2����2SO2����2H2O ����ʯ��ˮ c D�й����ɺڱ���E�й����ɰױ��� ace
CO2����2SO2����2H2O ����ʯ��ˮ c D�й����ɺڱ���E�й����ɰױ��� ace
����������1�����������������У�Ũ�����н�ǿ����������ʹ�����ۻ���ֹ��Ӧ��һ����������ȷ�����������汻�ۻ���
��2��������������ʹ���Ը��������ɫ����Һ���Ѿ������������ӣ�ѡ��a����ɸ��ţ�b�ܼ������������ӵĴ��ڣ�ѡc�������ֳ���������������������ɫ��Ӱ�����ֱ棻Fe2+������KMnO4��Һ����������ԭ��Ӧ��Fe2+��ʹ����KMnO4��Һ��ɫ����ȷѡ��d��
��SO2���л�ԭ�ԣ�ͨ��������ˮ�У�����SO2+Br2+2H2O=2HBr+H2SO4�����ɵ����������Ȼ����������ɫ��������n��������壩=0.448L��22.4L/mol=0.02mol�����ݶ�Ӧ��ϵ��SO2��BaSO4�� n��SO2��=n��BaSO4��=2.33g��233g/mol=0.01 mol�����n��SO2��=0.01mol�����Զ���������������Ϊ��0.01mol��0.02mol��100%=50%����ȷ����50%��
��3��A��ȥ����������������ʹƷ����Һ��ɫ������B���Լ���A���Ƿ���ȫ��ȥ������������ȷ��������SO2�Ƿ������
��4���ڼ���ʱ�������в�������Ũ���ᷴӦ��̼Ҳ��Ũ���ᷴӦ�������ɶ�����������̼��ˮ����Ӧ����ʽΪ��C��2H2SO4(Ũ)![]() CO2����2SO2����2H2O����ȷ����C��2H2SO4(Ũ)
CO2����2SO2����2H2O����ȷ����C��2H2SO4(Ũ)![]() CO2����2SO2����2H2O��
CO2����2SO2����2H2O��
��5��QΪ������̼��������̼�Ͷ���������ʹ����ʯ��ˮ����ǣ�ѡ��a��b�ܶ��������Ӱ�����ж϶�����̼�Ĵ��ڣ�ѡdʱ������̼����ʯ�����գ�B��C��������ʯ��ˮ�ɼ���CO2����ȷ��������ʯ��ˮ��c��
��6��������ԭ����ͭ������ˮ������ʹ��ɫ������ͭ��ĩ����ɫ��ͬʱ�к�ɫ��ͭ�������ɣ���ȷ�𰸣�D�й����ɺ�ɫ����E�й����ɰױ�����
��7��CO2���أ�O��=32/44��100%=72%, SO2���أ�O��=32/64��100%=50%����ij���������CO2��SO2��H2�е�һ�ֻ������ɣ����ⶨ����������������Ϊ50%�������������ֻ��SO2��������������Ϊ50%��a��ȷ��H2��SO2���������������������С��50%��b������H2��CO2�������������������������Ϊ50%��c��ȷ��CO2��SO2������������������������50%��d������SO2��CO2��H2������������������������Ե���50%��e��ȷ����ȷѡ��ace��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ��ݴ�����˵������������ ��
���ữѧ��(CP)
(500mL)
Ʒ��������
��ѧʽ��H2SO4
��Է���������98
�ܶȣ�1.84g/cm3
����������98%
A. �������Լ������ʵ���Ũ��Ϊ18.4mol��L-1
B. ��������������ˮ���������Һ��������������49%
C. ����200mL 4.6mol��L-1��ϡ������ȡ������62.5mL
D. ������,��2.7gAlͶ�������������пɵõ���״���µ�����3.36L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����Ũ�Ⱦ�Ϊ0.1mol/L ������ֱ�ΪVa��Vb��HA��Һ��BOH��Һ����ͬ����Ȼ�ϣ�����Va+Vb=100mL��Va��Vb����Һ��pH�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��
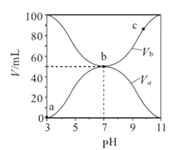
A. Ka(HA)��ֵ��Kb(BOH)��ֵ�����
B. b��ʱ��ˮ�������c(H+)=10-7 mol/L
C. c��ʱ��c(A-)>c(B+)
D. a��c������ ![]() ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء��Իش��������⣺
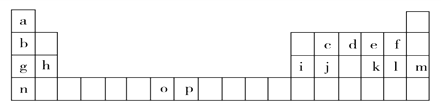
��1����̬oԭ�ӵ���Χ�����Ų�ͼ______________����̬p3+�����������Ų�ʽ_________��n��ԭ�ӽṹʾ��ͼ__________����̬jԭ�ӵĺ���������ʱ����ܼ�����Ϊ_______��
������Ԫ���У�û��δ�ɶԵ��ӵ�Ԫ����______�֡�
��2��ԭ������Ϊ52��Ԫ��x��Ԫ�����ڱ���������______________Ԫ����ͬһ�壨��д���ϱ�����ĸ��Ӧ��Ԫ�ط��ţ���
��3���ϱ���o��p������ĸ��ʾ��Ԫ�صĵ��������ֱܷ�ΪI3(o)��I3(p)����I3(o)__I3(p)������>����<������������_____________________________��
��4�����������ڱ���g��h��i��j����Ԫ�صĵ縺���ɴ�С����____________________����Ԫ�ط��ű�ʾ������һ�������ɴ�С����______________����Ԫ�ط��ű�ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������E��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ���ϳ�·�����£�

��1��A�Ľṹ��ʽΪ__________________________ ��
B�к�������������Ϊ______________��_______________��
��2��Cת��ΪD�ķ�Ӧ������_______________��
��3��д��D������NaOH��Һ��ȫ��Ӧ�Ļ�ѧ����ʽ___________________________��
��4��1molE������________molH2�ӳɡ�
��5��д��ͬʱ��������������B��һ��ͬ���칹��Ľṹ��ʽ __________________��
A���ܷ���������Ӧ B���˴Ź�������ֻ��4����
C������FeCl3��Һ������ɫ��Ӧ��ˮ��ʱ1mol������3molNaOH
��6����֪��ҵ�����ȱ�ˮ����ȡ���ӣ������ǻ�һ�㲻��ֱ�������������������ᱽ������![]() ����һ����Ҫ���л��ϳ��м��塣��������ϳ�·�����й���Ϣ����д���Ա����ױ�Ϊԭ����ȡ�����ᱽ�����ĺϳ�·������ͼ����ԭ����ѡ�����ϳ�·������ͼʾ�����£�
����һ����Ҫ���л��ϳ��м��塣��������ϳ�·�����й���Ϣ����д���Ա����ױ�Ϊԭ����ȡ�����ᱽ�����ĺϳ�·������ͼ����ԭ����ѡ�����ϳ�·������ͼʾ�����£�
![]() _________________________________________________________________________
_________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA���������ӵ�������ֵ,����˵������ȷ����(����)
A. lmolNa2O2������ˮ��Ӧ��ת�Ƶĵ�����ΪNA
B. ��״���£�2.24LCl2���������۷�Ӧת�Ƶĵ�����Ϊ0.2NA
C. 6.0g�������躬�е�Si-O����Ϊ0.4NA
D. 2mol���ڿ�������ȫȼ�գ�����O2�ķ�����Ϊ3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij�л���A�ķ����У����з��ǻ������ǻ����Ȼ��ȹ����ţ���ṹ��ʽ��ͼ��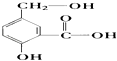
��ͬѧ�Ǹ��ݹ�����
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�ǣ�
��A��NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽ�ǣ�
��A��һ�������¸�Na��Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������100ml1mol/LCaCl2��Һ��������ȷ����
A. Cl-�����ʵ���Ũ��Ϊ2mol/L
B. CaCl2�����ʵ���Ϊ1mol
C. ȡ��50 mL��ʣ����Һ��CaCl2�����ʵ���Ũ��Ϊ0.5mol/L
D. ����Һ��ˮ�����Ϊ100 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ⱥ����ܱ�������ͨ��SO2��NO2��һ��������ʹ��ӦSO2(g)��NO2(g) ![]() SO3(g)��NO(g)�ﵽƽ�⣬����Ӧ������ʱ��仯��ͼ��ʾ����ͼ�ɵó�����ȷ�Ľ�����(����)
SO3(g)��NO(g)�ﵽƽ�⣬����Ӧ������ʱ��仯��ͼ��ʾ����ͼ�ɵó�����ȷ�Ľ�����(����)

A. ��Ӧ��H<0
B. ��Ӧ��Ũ��a ����� b ��
C. ��Ӧ��c��ﵽƽ��״̬
D. SO2 ��ת���ʣ�a��b ��С�� b��c ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com