

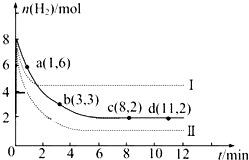
·ÖĪö ŗ£“ų¾×ĘÉÕµĆµ½ŗ£“ų»Ņ£¬½«Šü×ĒŅŗ·ÖĄėĪŖ²ŠŌüŗĶŗ¬µāĄė×ÓČÜŅŗӦєŌń¹żĀĖµÄ·½·Ø£®ŗ¬µāĄė×ÓČÜŅŗĶØČėŹŹĮæCl2ŹĒĪŖĮĖ½«µāĄė×ÓŃõ»Æ³Éµ„ÖŹµā£¬Ąė×Ó·½³ĢŹ½ĪŖ2I?+Cl2=I2+2Cl-£»ĄūÓƵāŅ×ČÜÓŚÓŠ»śČܼĮµÄŠŌÖŹĄ“½ųŠŠĢįČ”£¬×īŗóÓĆÕōĮóµÄ·½·Ø·ÖĄėµāŗĶÓŠ»śĪļ£¬ŅŌ“Ė½ā“šøĆĢā£®
½ā“š ½ā£ŗŗ£“ų¾×ĘÉÕµĆµ½ŗ£“ų»Ņ£¬½«Šü×ĒŅŗ·ÖĄėĪŖ²ŠŌüŗĶŗ¬µāĄė×ÓČÜŅŗӦєŌń¹żĀĖµÄ·½·Ø£®ŗ¬µāĄė×ÓČÜŅŗĶØČėŹŹĮæCl2ŹĒĪŖĮĖ½«µāĄė×ÓŃõ»Æ³Éµ„ÖŹµā£¬Ąė×Ó·½³ĢŹ½ĪŖ2I?+Cl2=I2+2Cl-£»ĄūÓƵāŅ×ČÜÓŚÓŠ»śČܼĮµÄŠŌÖŹĄ“½ųŠŠĢįČ”£¬×īŗóÓĆÕōĮóµÄ·½·Ø·ÖĄėµāŗĶÓŠ»śĪļ£¬
£Ø1£©×ĘÉÕŗ£“ųŠčŅŖ¼ÓČČ×°ÖĆ£¬ĖłŅŌ²½Öč¢Ł×ĘÉÕŗ£“ųŹ±£¬ŠčŅŖÓƵ½Čż½Å¼Ü£¬ŪįŪö£¬ÄąČż½ĒĶāŅŌ¼°¾Ę¾«µĘ£¬¹Ź“š°øĪŖ£ŗB£»
£Ø2£©²½Öč¢ŪŹĒ·ÖĄė¹ĢĢåŗĶŅŗĢ壬ŌņŹµŃé²Ł×÷ĪŖ¹żĀĖ£¬²½Öč¢ŽµÄÄæµÄŹĒ“Óŗ¬µā±½ČÜŅŗÖŠ·ÖĄė³öµ„ÖŹµāŗĶ»ŲŹÕ±½£¬
²½Öč¢ŻŹĒĢįČ”µāĖ®ÖŠµÄµā£¬ŅņµāŅ×ČÜÓŚ±½µČÓŠ»śČܼĮ£¬æÉÓĆŻĶČ”·ÖŅŗµÄ·½·Ø·ÖĄė£¬
¹Ź“š°øĪŖ£ŗ¹żĀĖ£»ŻĶČ”·ÖŅŗ£»
£Ø3£©µāĄė×ÓŌŚĖįŠŌĢõ¼žĻĀæɱ»MnO2Ńõ»ÆÉś³Éµ„ÖŹµā£ŗĄė×Ó·½³ĢŹ½ĪŖ£ŗ2I-+MnO2+4H+=Mn2++I2+2H2O£¬¹Ź“š°øĪŖ£ŗ2I-+MnO2+4H+=Mn2++I2+2H2O£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄ·ÖĄė”¢Ģį“æŅŌ¼°ÖʱøŹµŃé·½°øµÄÉč¼Ę£¬ĪŖøßĘµæ¼µć£¬²ąÖŲӌѧɜµÄ·ÖĪö”¢ŹµŃéÄÜĮ¦µÄ漲飬ĢāÄæÄѶČÖŠµČ£¬×¢Ņā»ł±¾ŹµŃé²Ł×÷µÄŅŖµćŗĶ×¢ŅāŹĀĻī£®


 Äæ±ź²āŹŌĻµĮŠ“š°ø
Äæ±ź²āŹŌĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ś”¢¢Ū»ņ¢Ü | B£® | ¢Ś”¢¢Ū»ņ¢Ż | C£® | ¢Ł”¢¢Ū»ņ¢Ż | D£® | ¢Ł”¢¢Ū»ņ¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĀČĖį¼ŲŹÜČČ·Ö½āÖĘŃõĘų | B£® | ĀĮČČ·“Ó¦ | ||
| C£® | ÄĘŗĶĖ®·“Ó¦ | D£® | ŹµŃéŹŅÖʱøĒāĘų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ¹¤ŅµÉĻ”°¹Ģ¶Ø”±ŗĶĄūÓĆCO2ÄÜÓŠŠ§µŲ¼õĒį”°ĪĀŹŅ”±Š§Ó¦£¬æÉÓĆCO2Éś²śČ¼ĮĻ¼×“¼£ŗ
¹¤ŅµÉĻ”°¹Ģ¶Ø”±ŗĶĄūÓĆCO2ÄÜÓŠŠ§µŲ¼õĒį”°ĪĀŹŅ”±Š§Ó¦£¬æÉÓĆCO2Éś²śČ¼ĮĻ¼×“¼£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  Ļņ1LÅØ¶Č¾łĪŖ0.1mol/LµÄBa£ØOH£©2”¢NaAlO2»ģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė0.1mol/lŹĒH2SO4ČÜŅŗ | |
| B£® | 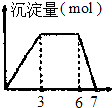 Ļņŗ¬0.1mol/LµÄAlCl3ŗĶ0.3mol/LNH4ClµÄ1L»ģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė0.1mol/lŹĒNaOHČÜŅŗ | |
| C£® | 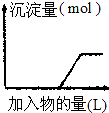 ĻņÉÕ¼īČÜŅŗÖŠÖšµĪ¼ÓČėĆ÷·ÆČÜŅŗ | |
| D£® | 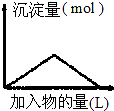 ĻņBa£ØOH£©2ČÜŅŗÖŠÖš½„ĶØČė¶žŃõ»ÆĢ¼ĘųĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | N2ŗĶH2“߻ƼÓČČÖĘČ”°±Ęų | B£® | ¼ÓČČNH4ClÖĘČ”°±Ęų | ||
| C£® | ½«ÅØ°±Ė®ĻņŃõ»ÆøĘ¹ĢĢåÉĻµĪ¼Ó | D£® | ½«NH4ClČÜŅŗŗĶNaOHČÜŅŗ»ģŗĻ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com