
| |||||||||||||||||||||||
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������38�ס�2008ȫ����ʡ�и߿�ģ��������(��ٰ�)������ѧ ��ٰ� ���ͣ�022
�˵������С����˳�����е����ֶ�����Ԫ��X��Y��Z��W��Q�����У���ͬ����Ԫ���У�Z�Ľ�������ǿ��Q�ķǽ�������ǿ��W�ĵ����ǵ���ɫ���壻X��Y��W�����ڱ��е����λ�ù�ϵ��ͼ��ʾ��

(1)����Ԫ���γɵĵ��ʣ����У���̬ʱ����ԭ�Ӿ������________(�ѧʽ)��������ǿ����������________��________(�ѧʽ)��
(2)������Ԫ���У�ԭ�Ӱ뾶������________(�ѧʽ)����Z��W��Q�γɼ����Ӱ뾶�ɴ�С��˳����________(�û�ѧʽ��ʾ)��X��W��Q������������ˮ�������Դ�ǿ������˳��Ϊ________(�û�ѧʽ��ʾ)��
(3)Y��Z�γɵ�һ�ֻ������к������Ӽ����ۼ���д���û�������ˮ��Ӧ�Ļ�ѧ����ʽ________��
(4)Z��Q��Ӧ���ɵĻ���������________��������û�����ı�����Һ�����ĵ缫��ӦʽΪ________�����һ��ʱ������������������Һ��Ϸ�Ӧ�Ļ�ѧ����ʽ��________��
(5)��X��Y��������Ԫ����ɵĻ�����X6H12Y6����֪��9 g�û�����ȼ������XY2��Һ̬H2Yʱ���ų�140 kJ���ȣ�д���û�����ȼ���ȵ��Ȼ�ѧ����ʽ��________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10�꽭����У��һ��ѧ������������ѧ�� ���ͣ�ѡ����
��a��b��c��d��������Ԫ�أ���֪a��b�������Ӻ�c��d�������Ӷ�������ͬ�ĵ��Ӳ�ṹ������ԭ�Ӱ뾶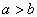 �������������ĸ������Ϊ
�������������ĸ������Ϊ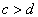 ��������Ԫ�غ˵������С�����˳��Ϊ��
��
��������Ԫ�غ˵������С�����˳��Ϊ��
��
A. 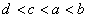 B.
B. 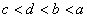 C.
C.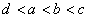 D.
D. 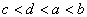
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0107 ģ���� ���ͣ��ƶ���
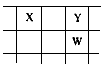
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com