
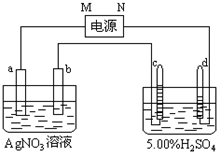
 ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�����Ϲ��ռ���336mL����״̬�����壮�ش�
ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�����Ϲ��ռ���336mL����״̬�����壮�ش�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
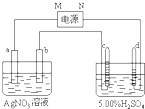 ��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ���ֻ��c��d�����Ϲ��ռ���336mL����״̬�����壮�ش�
��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ���ֻ��c��d�����Ϲ��ռ���336mL����״̬�����壮�ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
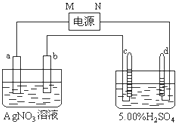 ��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ���b�缫��֣�����c��d�缫����������������Ϲ��ռ���336mL����״̬�����壮�ش�
��ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ���b�缫��֣�����c��d�缫����������������Ϲ��ռ���336mL����״̬�����壮�ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
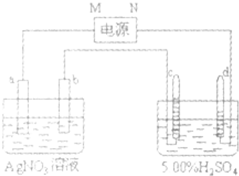 ͼ�е缫a��b�ֱ�ΪAg�缫��Pt������缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�������ռ���336mL����״�������壬�������Ϊ1��2���ش�
ͼ�е缫a��b�ֱ�ΪAg�缫��Pt������缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�������ռ���336mL����״�������壬�������Ϊ1��2���ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��11�֣�ͼ�е缫a��b�ֱ�ΪAg�缫��Pt������缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�������ռ���336mi������״�������壬�������Ϊl��2���ش�
��1��ֱ����Դ�У�MΪ ����
��2��b�缫�ϵĵ缫��Ӧ����ʽ ��c�缫�ϵĵ缫��Ӧ����ʽ ��
��3��ͨ��һ��ʱ�䣬AgNO3��Һ��pH�����������С�����䡱���� ��
��4����ͨ��һ��ʱ���H2SO4��Һ���ʴ������5��00����Ϊ5��02������ԭ5��00������H2SO4��ҺΪ g����������λ��Ч���֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com