
Ϋώ”–HAΓΔH2BΝΫ÷÷»θΥαΘ§”–»γœ¬ΙΊœΒΘΚH2B+AΘ≠ΘΫHBΘ≠+HA B2-+HAΘΫHBΘ≠+AΘ≠‘ρœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «ΘΚ
AΓΔH2BΒΡΒγάκΖΫ≥Χ ΫΈΣΘΚH2B 2H+ + B2Θ≠
2H+ + B2Θ≠
BΓΔΫαΚœ÷ Ή”ΡήΝΠ”…«ΩΒΫ»θΒΡΥ≥–ρΈΣΘΚB2Θ≠>AΘ≠>HBΘ≠
CΓΔΒ»Έο÷ ΒΡΝΩ≈®Ε»NaAΓΔNaHBΓΔNa2B»ΐ÷÷―ΈΒΡΥ°»ή“ΚΘ§NaAΦν–‘Ήν«Ω
DΓΔΒ»pHΒΡHAΓΔH2BΝΫ÷÷»ή“Κ÷–Θ§Υ°ΒΡΒγάκ≥ωΒΡ«βάκΉ”«Α’Ώ¥σ”ΎΚσ’Ώ
B
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚΖ¥”Π1Ω…“‘Ω¥ΒΫΘ§H2B-<HA(Βγάκάμ¬έΘ©Θ§Υυ“‘Υα–‘HAΘΨH2C->HC2- Ζ¥”Π2÷–HB-<HAΘ®Ά§…œΘ©ΓΘ“ρΈΣB2-ΒΡΦν–‘Ήν«ΩΘ§÷ Ή”Υ°Ϋβ“ΜΑψœ‘Υα–‘«“B2-ΒΡ¥χΒγΝΩΉν¥σΘ§Ήν»ί“ΉΈϋ“ΐ÷ Ή”Θ§HB-ΒΡΥα–‘Ήν«ΩΘ§÷ Ή”Υ°Ϋβ“ΜΑψœ‘Υα–‘«“HB-ΒΡ¥χΒγΝΩΉν–ΓΘ§Έϋ“ΐ÷ Ή”ΒΡΡήΝΠΫœ–ΓΓΘΙ ―ΓBΓΘ
ΩΦΒψΘΚ»θΒγΫβ÷ ΒΡΒγάκ
ΒψΤάΘΚ±ΨΧβΩΦ≤ιΒΡ «»θΒγΫβ÷ ΒΡΒγάκΘ§¥”ΖΫ≥Χ Ϋ÷–±»ΫœΘ§»ί“ΉΒΟ÷ Ή”ΒΡάκΉ”Θ§Φν–‘«ΩΘ§Ζ¥÷°Θ§Υα–‘«ΩΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2012-2013―ßΡξΝ…Ρΰ ΓΒΛΕΪ –ΩμΒιΕΰ÷–ΗΏΕΰ…œ―ßΤΎΤΎΡ©ΩΦ ‘Μ·―ß ‘ΨμΘ®¥χΫβΈωΘ© Χβ–ΆΘΚΒΞ―ΓΧβ
Ϋώ”–HAΓΔH2BΝΫ÷÷»θΥαΘ§”–»γœ¬ΙΊœΒΘΚH2B+AΘ≠ΘΫHBΘ≠+HA B2-+HAΘΫHBΘ≠+AΘ≠‘ρœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «ΘΚ
AΓΔH2BΒΡΒγάκΖΫ≥Χ ΫΈΣΘΚH2B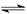 2H+ + B2Θ≠
2H+ + B2Θ≠
BΓΔΫαΚœ÷ Ή”ΡήΝΠ”…«ΩΒΫ»θΒΡΥ≥–ρΈΣΘΚB2Θ≠>AΘ≠>HBΘ≠
CΓΔΒ»Έο÷ ΒΡΝΩ≈®Ε»NaAΓΔNaHBΓΔNa2B»ΐ÷÷―ΈΒΡΥ°»ή“ΚΘ§NaAΦν–‘Ήν«Ω
DΓΔΒ»pHΒΡHAΓΔH2BΝΫ÷÷»ή“Κ÷–Θ§Υ°ΒΡΒγάκ≥ωΒΡ«βάκΉ”«Α’Ώ¥σ”ΎΚσ’Ώ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
―–ΨΩΜ·―ßΖ¥”Π Β÷ Θ§ΧΫΨΩΖ¥”ΠΙφ¬…Θ§ «ΧαΗΏΈ“Ο«Μ·―ßΥΊ―χΒΡ”––ß ÷ΕΈΘ§Τδ÷–ΓΑ“‘«Ω÷Τ»θΓ±ΒΡΙφ¬… «Έ“Ο«Υυ λ÷ΣΒΡΓΘ
Θ®1Θ©Ϋώ”–HAΓΔH2BΝΫ÷÷»θΥαΘ§”–»γœ¬ΙΊœΒΘΚH2BΘ®…ΌΝΩΘ©+A-=== HB-+HA Θ§‘ρ‘ΎA-ΓΔHB-ΓΔB2-άκΉ”÷–Θ§Ήν“ΉΫαΚœ÷ Ή”ΒΡ « ΘΜ
Θ®2Θ©Υα–‘«Ω»θ≥ΐΝΥ”κΈο÷ ±Ψ…μ τ–‘”–ΙΊΆβΘ§ΜΙΚΆ»ήΦΝ”–ΙΊΘ§»γCH3COOH”κHF‘Ύ“ΚΑ±÷– ήNH3”ΑœλΩ…ΖΔ…ζΆξ»ΪΒγάκΓΘΡ«Ο¥‘Ύ“ΚΑ±÷–Ζ¥”ΠΘΚCH3COONa + HCl == NaCl + CH3COOH ΡήΖώΖΔ…ζΘ§ΈΣ ≤Ο¥ΘΩ ΘΜ
Θ®3Θ©¬ΝοßΖ·Θ§Τδ÷ς“ΣΜ·―ß≥…Ζ÷ΈΣ °ΕΰΥ°ΝρΥα¬ΝοßΘ§œρΗΟ―ΈΒΡ»ή“Κ÷–÷πΒΈΦ”»κNaOH»ή“ΚΘ§≤ζ…ζΒΡœ÷œσ”–ΘΚ
ΔΌ»ή“Κ÷–≥ωœ÷ΑΉ…Ϊ≥ΝΒμΘΜ
ΔΎ”–¥ΧΦΛ–‘ΤχΈΕΒΡΤχΧεΖ≈≥ωΘΜ
ΔέΑΉ…Ϊ≥ΝΒμΝΩ÷πΫΞ‘ωΕύΘΜ
ΔήΑΉ…Ϊ≥ΝΒμ÷πΫΞœϊ ßΘΜ
«κ≈≈≥ω“‘…œΗς÷÷œ÷œσ”…œ»ΒΫΚσ≥ωœ÷ΒΡ’ΐ»ΖΥ≥–ρΘ®”Ο–ρΚ≈ΜΊ¥πΘ© ΘΜ
Θ®4Θ©‘Ύ―θΜ·ΜΙ‘≠Ζ¥”Π÷–Θ§“≤”–ΓΑ“‘«Ω÷Τ»θΓ±ΒΡάύΥΤΙφ¬…ΓΘ“―÷ΣMnO2‘ΎΥα–‘»ή“Κ÷–“Ή±ΜΜΙ‘≠≥…Mn2+Θ§ MnO2ΓΔCl2ΓΔFeCl3ΓΔI2ΒΡ―θΜ·–‘“ά¥ΈΦθ»θΓΘœ¬Ν–Ζ¥”Π‘ΎΥ°»ή“Κ÷–≤ΜΩ…ΡήΖΔ…ζΒΡ «( )
AΘ°3Cl2 ΘΪ 6FeI2 = 2FeCl3 ΘΪ4FeI3 BΘ°Cl2 ΘΪ FeI2=FeCl2 ΘΪI2
CΘ°MnO2ΘΪ4HCl = MnCl2 ΘΪCl2ΓϋΘΪ2H2O DΘ°2Fe3ΘΪΘΪ2IΘ≠= 2Fe2ΘΪΘΪI2
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com