
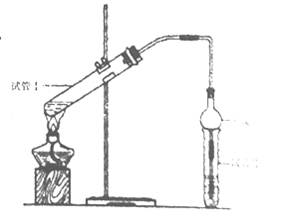
| ʵ���� | �Թܢ��е��Լ� | �Թܢ��е��Լ� | ����л���ĺ��/cm |
| A | 2mL�Ҵ���2 mL���ᡢ1 mL 18mol/LŨ���� | ����̼������Һ | 5.0 |
| B | 3 mL�Ҵ���2 mL���� | 0.1 | |
| C | 3 mL�Ҵ���2 mL���ᡢ6 mL 3mol/L���� | 1.2 | |
| D | 3 mL�Ҵ���2 mL���ᡢ���� | 1.2 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
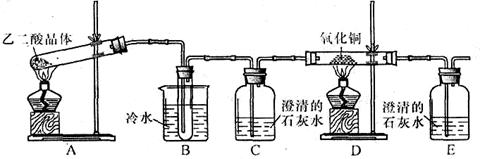
 �������Ͽ�֪���Ҷ��ᾧ��(H2C2O4��2H2O)�۵�100��1 �棬�������������ˮ�İ�ɫ���壻Cu2O������ϡ���ᣬ���������绯��Ӧ����Cu2+��Cu��
�������Ͽ�֪���Ҷ��ᾧ��(H2C2O4��2H2O)�۵�100��1 �棬�������������ˮ�İ�ɫ���壻Cu2O������ϡ���ᣬ���������绯��Ӧ����Cu2+��Cu�� ��һ��̽����
��һ��̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

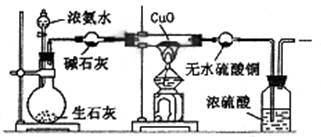
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ô�п��ϡ���ᷴӦ | B���ú�ͭ�ȵĴ�п��ϡ���ᷴӦ |
| C���ô�п��Ũ���ᷴӦ | D���ú�ͭ�ȵĴ�п��ϡ���ᷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
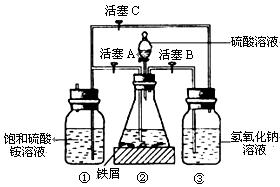
| A�������ſ����� | B�������ſ����� | C����ˮ�� | D�������ϴ������ռ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2CuO,CuO+2HNO3="=" Cu(NO3)2 + H2O
2CuO,CuO+2HNO3="=" Cu(NO3)2 + H2O 3Cu(NO3)2��2NO����4H2O
3Cu(NO3)2��2NO����4H2O| A�����ַ��������漰�ķ�Ӧ������������ԭ��Ӧ |
| B�����ַ���������������ʣ���>��>�� |
| C����ȡ��ͬ��������ͭ���ڲ������к�����Ȣ۶� |
| D�����ַ����У��ٷ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


| A������Kͨ��N2���Թ�A�������ݲ�����˵��װ������������ |
| B��U�ι���ʢ�ŵĸ�����ȿ����ü�ʯ�ң�Ҳ������Ũ���� |
| C����Ӧ��������Ϩ��ƾ��ƣ�����Ӧ����ȴ���ٹرջ���K |
| D��������Ca3N2���������У�ֻ�ܵõ�һ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com