
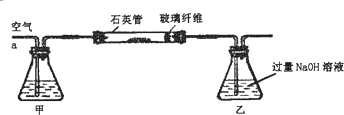
���� ��1��Ϊ��ֹ�������Һ�л���BaCO3������Ӱ��ʵ������Ӧ�������еĶ�����̼��ȥ�����Լ�װ���п���NaOH��Һ������NaOH��Һ��SO2��Ӧ����SO32-��H2O��������������ܱ���������������������ӣ�
��2�������������ǿ�����ԣ������������������Ϊ�������ͬʱ����ˮ��
��3�����յõ��ij���Ϊ���ᱵ��������ԭ���غ����������������������Ӷ�����������������
��4������250mlһ�����ʵ���Ũ�ȵ���Һ��Ҫ�ձ�������������ͷ�ιܡ���Ͳ��250ml����ƿ��������
��5�����һ�θ��������Һ����ʱ����Һ��ɫͻ��Ϊ��ɫ������30s�ڲ���ɫ��˵���ζ������յ㣻
��6��������������Ԫ�ص���������Ϊa�����ݹ�ϵʽMnO4-��5Fe2+��5Fe��������������Ԫ�ص�����������
��� �⣺��1��Ϊ��ֹ�������Һ�л���BaCO3������Ӱ��ʵ������Ӧ�������еĶ�����̼��ȥ�����Լ�װ���п���NaOH��Һ������NaOH��Һ��SO2��Ӧ����SO32-��H2O��������������ܱ���������������������ӣ����з�Ӧ���ӷ���ʽΪSO2+2OH-=SO32-+H2O\2SO32-+O2=2SO42-��
�ʴ�Ϊ��NaOH��SO2+2OH-=SO32-+H2O��2SO32-+O2=2SO42-��
��2�������������ǿ�����ԣ������������������Ϊ�������ͬʱ����ˮ����Ӧ���ӷ���ʽΪ��SO32-+H2O2�TSO42-+H2O��
�ʴ�Ϊ��SO32-+H2O2�TSO42-+H2O��
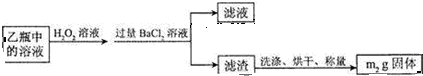
��3��������Ԫ���غ㣬m1gFeS2�е���Ԫ������ȫ������BaSO4������n��BaSO4��=$\frac{m{\;}_{2}}{233}$mol������n��S��=$\frac{m{\;}_{2}}{233}$mol�����Ի���������Ԫ�ص���������Ϊ��$\frac{\frac{{m}_{2}}{233}mol��32g/mol}{m{\;}_{1}}$��100%=$\frac{32m{\;}_{2}}{233m{\;}_{1}}$��100%��
�ʴ�Ϊ��$\frac{32m{\;}_{2}}{233m{\;}_{1}}$��100%��
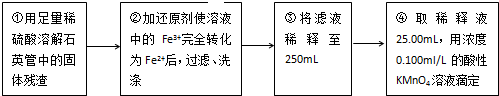
��4����IJ�����У�һ�����ʵ���Ũ����Һ�������У������ձ�������������ͷ�ι��⣬����Ҫ250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
��5�����һ�θ��������Һ����ʱ����Һ��ɫͻ��Ϊ��ɫ������30s�ڲ���ɫ��˵���ζ������յ㣬
�ʴ�Ϊ�����һ�θ��������Һ����ʱ����Һ��ɫͻ��Ϊ��ɫ������30s�ڲ���ɫ��
��6��������������Ԫ�ص���������Ϊa����
MnO4-��5Fe2+��5Fe
1mol 5��56g
0.1mol/L��25��10-3L��10 m1a g
����1mol��0.1mol/L��25��10-3L��10=5��56g��m1a g
���a=$\frac{7}{m{\;}_{1}}$��
�ʴ�Ϊ��$\frac{7}{m{\;}_{1}}$��
���� ���⿼�����ʳɷֺͺ����IJⶨ���漰���û�ѧ�����Һ�����ơ�������ԭ�ζ�����ѧ����ȣ����н�ǿ���ۺ��ԣ�����ʱע��������غ����ϵʽ�ĽǶȷ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{m}{a}L$ | B�� | $\frac{2m}{3a}L$ | C�� | $\frac{m+n}{a}L$ | D�� | $\frac{2��m+n��}{3a}L$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuSO4•3H2O | B�� | CuSO4•2H2O | C�� | CuSO4•H2O | D�� | CuSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3COOH+CH3CH2OH $?_{��}^{Ũ����}$CH3COOCH2CH33+H2O | |
| B�� | CH2�TCH2+HBr��CH3CH2Br | |
| C�� | CH4+Cl2$\stackrel{��}{��}$CH3Cl+HCl | |
| D�� | 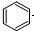 +Br2$\stackrel{FeBr_{3}}{��}$ +Br2$\stackrel{FeBr_{3}}{��}$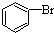 +HBr +HBr |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2SO4��NaHSO4�������� | B�� | ���ʹ�����ڼ� | ||
| C�� | KOH��NH3•H2O�����ڵ���� | D�� | Na2O��Na2O2�����ڼ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
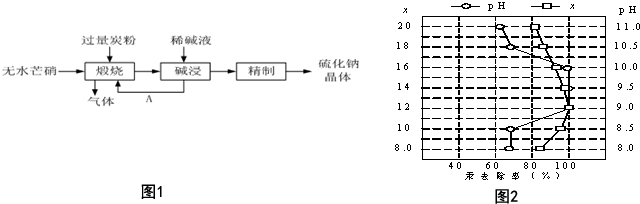
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 9��4 | B�� | 6��1 | C�� | 7��6 | D�� | 11��6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
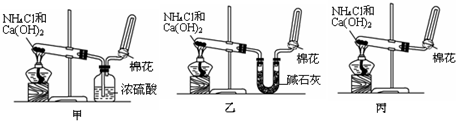
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com