

| ||
| ||
| ||
| ||


 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2012�������ǰ��Ϣ����ѧ���� ���ͣ�058
��������������IJ�ҩ��Ŀɰ�������Ҫ�м��壮��������ˮ����ػ���ˮ̼��صĴ����£��Ա���ȩ�ʹ�����Ϊԭ���Ʊ���

�Ա���ȩ�ʹ�����Ϊ��Ҫԭ���Ʊ�������ʵ�鲽��Ϊ��
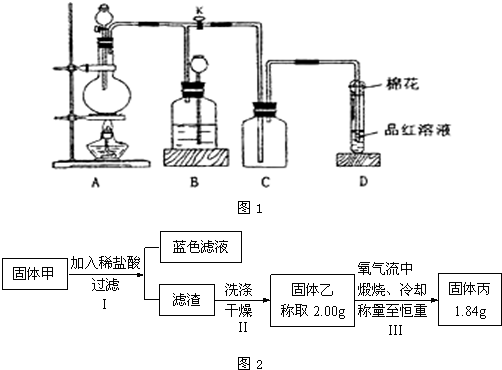
����100 mL�����Բ����ƿ������1.5 mL��������ı���ȩ��4 mL��������Ĵ������Լ���ϸ��2.2 g��ˮ̼��ء�2����ʯ����ͼl��ʾ���Ӻ�װ�ã�
�ڼ��Ȼ���(С�����)40 min��������С����ʹ��Һ�պû�����
��ֹͣ���ȣ�����Ӧ����ȴ��
������ˮ�ܽⷴӦ������ͼ2��ʾװ�ý���ˮ��������10��NaOH�ܽ⡢����̿��ɫ����ȴ�õ���ɫ�ֲ�ƷA��
����ˮ���Ҵ��ؽᾧ�ð�ɫ����B��

(1)��������װ��Ҫ���ԭ����________��
(2)����ʱ����Ӧ�¶Ȳ���̫�ߵ�ԭ����________��
(3)��ʵ����Ҫ����õ����ˣ������йس��˵�˵���У���ȷ����________��(����ĸ)
A������ʱ����ֽ�Ĵ�СӦ�벼��©���ײ�ǡ��һ��
B������ǰ�����ܼ�����ֽʪ��ʹ��ֽ��©���ײ�����
C�����˽���ʱӦ�ȹس����ã���������ƿ�ӹ�
D�����������ó�����ʹ����ƿ�е�ѹǿ���ͣ��ﵽ��Һ�����Ŀ��
(4)��Ϊˮ������������װ����T�ܳ��Ⱦ����ܶ�һЩ���ҿ���ˮ����������һ����������б����������ԭ����________��
(5)��ɫ�ֲ�ƷA��������Ҫ����Ϊ________��
(6)��ijͬѧ�õ���ɫƬ״����1.2 g���Լ��������Ϊ________(����3λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
Ϊ̽��ͭ��Ũ����ķ�Ӧ��ij��ȤС�����������ʵ�顣
��ʵ��1��ͭ��Ũ���ᷴӦ��ʵ��װ����ͼ��ʾ��
(1)��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ��
(2)��Ϩ��ƾ��ƺ���Ϊ�е��ܴ��ڣ�B�е�Һ�岻�ᵹ������ԭ���ǣ� ��
���װ��ǰ����������Ϊ��ʹװ���еIJ���������ȫ�����գ�Ӧ����ȡ�IJ����ǣ� ��
��ʵ��2��ʵ���з����Թ��ڳ��˲�����ɫ�����⣬��ͭ˿���滹�к�ɫ��������ɣ����п��ܺ�������ͭ��������ͭ����ͭ������ͭ��
�������ϣ�
��������ͭ�����Ի����»ᷢ������������ԭ��Ӧ����Cu2+��ͭ���ʣ��ڿ����г�����գ�����ת��Ϊ����ͭ��
����ͭ������ͭ�����¶�������ϡ���ᣬ�ڿ����г�����գ���ת��Ϊ����ͭ�Ͷ�������
Ϊ���о��ijɷ֣���С��ͬѧ���ռ����㹻���Ĺ������������ʵ�飺
(3)��������м��������Ƿ�ϴ�Ӹɾ���ʵ�鷽���ǣ� ��
(4)���������ڿ���������ʱ��ʹ�õ�ʵ���������˲����������żܡ��ƾ����⣬�������У�
��
(5)��ȷ�����Ƿ�������ȫ�IJ����ǣ� ��
(6)�����չ�����һ�������Ļ�ѧ��Ӧ����ʽΪ�� ��
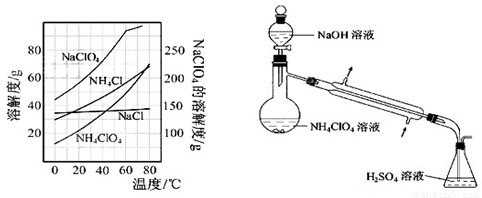
������泥�NH4ClO4���Ǹ��ϻ���ƽ�������Ҫ�ɷ֣�ʵ���ҿ�ͨ�����з�Ӧ��ȡ��
��������NaClO4(aq)+NH4Cl(aq) NH4ClO4(aq)+NaCl(aq)
(7)���ð�����Ũ�������NH4Cl��������Ӧ����Ҫ��繩�Ⱦ��ܽ��У���ԭ����:
��
(8)����Ӧ�õ��Ļ����Һ��NH4ClO4��NaCl�����������ֱ�Ϊ0.30��0.l5��������ʵ��ܽ����������ͼ�����ӻ����Һ�л�ý϶�NH4ClO4�����ʵ���������Ϊ����������ƣ�����Ũ���� �����ˣ� �����
Ϊ�˲ⶨ��Ʒ��NH4ClO4�ĺ�����װ������ͼ��ʾ������װ�á������̶�װ������ȥ����ʵ�鲽�����£�

(9)������3�У�ȷȡ��24.00 mL H2SO4(aq)�IJ��������� ��
(10)��ʵ����ȷ�����ɵİ���ϡ������ȫ���յ�ʵ�鲽���� ����д�����ţ���
(11)�������ظ�ʵ��2~3�Σ���ԭ���� ��
(12)�����0.320 g�����к�NH4ClO4��ȷֵΪ0.095g����ʵ��ⶨ�����0.092g ����ʵ��
��������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ���������������ѧ����ģ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ʵ������г��ֵ��쳣�������̽������ʵʩ���ʽ������������¾������Ч;����
��ʵ��1������ͼʵ��װ�ý���ͭ��Ũ����ķ�Ӧ��ʵ���з����Թ��ڳ��˲�����ɫ����ͭ�����⣬��ͭ˿���滹�к�ɫ��������ɣ����п��ܺ��к�ɫ��CuO��CuS��Cu2S���������ϣ�CuS��Cu2S��Ϊ��ɫ���壬�����¶�������ϡ���ᣬ�ڿ��������գ���ת��ΪCuO��SO2��Ϊ��̽���ijɷ֣���С��ͬѧ���ռ����㹻���Ĺ������������ʵ�飺


��1������ʵ��װ�ó�����β������װ���⣬����һ���������ŵ��� ��
��2���������ڿ���������ʱ��ʹ�õ�ʵ���������˲����������żܡ��ƾ����⣬�������� ��
��3��ȷ�����Ƿ�������ȫ�IJ����� ��
��4�����չ�����һ�������Ļ�ѧ��Ӧ����ʽΪ ��
��ʵ��2��ij��ѧ��ȤС�����������ʵ��װ�ã�β������װ��δ��������̽��Cl2��Br2��Fe3+��������ǿ����

��5����������ʵ�������������дʵ����ۡ�
|
ʵ����� |
ʵ������ |
���� |
|
����a����Բ����ƿ�е�������Ũ���Ȼ��رջ���a����ȼ�ƾ��ơ� |
Dװ���У���Һ��� Eװ���У�ˮ����Һ��ƣ� ��CCl4�������Ա仯�� |
Cl2��Br2��Fe3+����������ǿ������˳��Ϊ��
|
��6����æ�ڹ۲�ͼ�¼��û�м�ʱֹͣ��Ӧ��D��E�о��������쳣�ı仯��Dװ���У���ɫ������ȥ��Eװ���У�CCl4��������ɫ��Ϊ��ɫ������ɫ���ֱ����ɺ�ɫ��
Ϊ̽������ʵ������ı��ʣ�С��ͬѧ����������£�
|
����(SCN)2������±�ص������ơ������ԣ�Cl2��(SCN)2 �� ����Cl2��Br2��Ӧ���ɵ�BrCl�ʺ�ɫ���е�Լ5�棬��ˮ����ˮ�ⷴӦ�� ����AgClO��AgBrO��������ˮ�� |
������ƽ���ƶ�ԭ������ϻ�ѧ�������Cl2����ʱD����Һ��ɫ��ȥ��ԭ��____ ��
����Ƽ�ʵ��֤���������� ��
����̽��E����ɫ�仯��ԭ�����ʵ�����£��÷�Һ©�������E���²���Һ�������ռ���ɫ���ʣ�ȡ����������AgNO3��Һ������۲쵽���а�ɫ�������������ϻ�ѧ������ͽ�������ɫ������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ϻ���������������ѧ����ĩ������⻯ѧ�Ծ� ���ͣ�ʵ����
Ϊ̽��ͭ��Ũ����ķ�Ӧ��ij��ȤС�����������ʵ�顣
��ʵ��1��ͭ��Ũ���ᷴӦ��ʵ��װ����ͼ��ʾ��
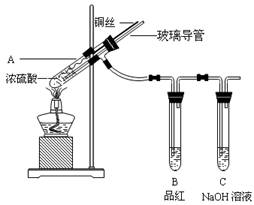
(1)��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ��
(2)��Ϩ��ƾ��ƺ���Ϊ�е��ܴ��ڣ�B�е�Һ�岻�ᵹ������ԭ���ǣ� ��
���װ��ǰ����������Ϊ��ʹװ���еIJ���������ȫ�����գ�Ӧ����ȡ�IJ����ǣ� ��
��ʵ��2��ʵ���з����Թ��ڳ��˲�����ɫ�����⣬��ͭ˿���滹�к�ɫ��������ɣ����п��ܺ�������ͭ��������ͭ����ͭ������ͭ��
�������ϣ�
��������ͭ�����Ի����»ᷢ������������ԭ��Ӧ����Cu2+��ͭ���ʣ��ڿ����г�����գ�����ת��Ϊ����ͭ��
����ͭ������ͭ�����¶�������ϡ���ᣬ�ڿ����г�����գ���ת��Ϊ����ͭ�Ͷ�������
Ϊ���о��ijɷ֣���С��ͬѧ���ռ����㹻���Ĺ������������ʵ�飺
(3)��������м��������Ƿ�ϴ�Ӹɾ���ʵ�鷽���ǣ� ��
(4)���������ڿ���������ʱ��ʹ�õ�ʵ���������˲����������żܡ��ƾ����⣬�������У�
��
(5)��ȷ�����Ƿ�������ȫ�IJ����ǣ� ��
(6)�����չ�����һ�������Ļ�ѧ��Ӧ����ʽΪ�� ��
������泥�NH4ClO4���Ǹ��ϻ���ƽ�������Ҫ�ɷ֣�ʵ���ҿ�ͨ�����з�Ӧ��ȡ��
��������NaClO4(aq)+NH4Cl(aq)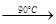 NH4ClO4(aq)+NaCl(aq)
NH4ClO4(aq)+NaCl(aq)
(7)���ð�����Ũ�������NH4Cl��������Ӧ����Ҫ��繩�Ⱦ��ܽ��У���ԭ����:
��
(8)����Ӧ�õ��Ļ����Һ��NH4ClO4��NaCl�����������ֱ�Ϊ0.30��0.l5��������ʵ��ܽ����������ͼ�����ӻ����Һ�л�ý϶�NH4ClO4�����ʵ���������Ϊ����������ƣ�����Ũ���� �����ˣ� �����
Ϊ�˲ⶨ��Ʒ��NH4ClO4�ĺ�����װ������ͼ��ʾ������װ�á������̶�װ������ȥ����ʵ�鲽�����£�
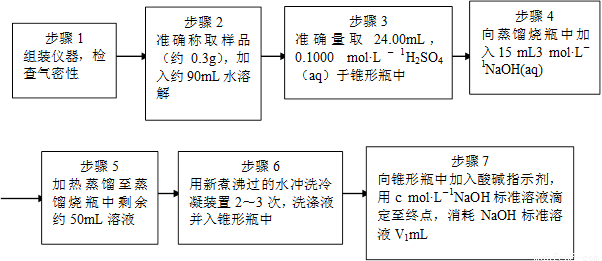
(9)������3�У�ȷȡ��24.00 mL H2SO4(aq)�IJ��������� ��
(10)��ʵ����ȷ�����ɵİ���ϡ������ȫ���յ�ʵ�鲽���� ����д�����ţ���
(11)�������ظ�ʵ��2~3�Σ���ԭ���� ��
(12)�����0.320 g�����к�NH4ClO4��ȷֵΪ0.095g����ʵ��ⶨ�����0.092g ����ʵ��
��������Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com