
ĻĀĮŠÓŠĮņ²ĪÓėµÄĖÄøö·“Ó¦¶¼ÓŠĢŲŹāµÄ×÷ÓĆ”£
¢ŁS+HgØTØTHgS£¬¢Ś3S+2Al![]() Al2S3£¬
Al2S3£¬
¢ŪS+2KNO3+3C![]() K2S+N2”ü+3CO2”ü£¬
K2S+N2”ü+3CO2”ü£¬
¢Ü3S+6KOHØTØT2K2S+K2SO3+3H2O£¬ĘäÖŠ¢Ł³£ÓĆÓŚĻū³żŹŅÄŚ¹ÆÕōĘųµÄĪŪČ¾£¬¢ŚÓĆÓŚÖʱøAl2S3£¬¢ŪŹĒĪŅ¹ś¹Å“śĖÄ“ó·¢Ć÷Ö®Ņ»”Ŗ”ŖŗŚ»šŅ©£ØŅ»Įņ¶žĻõČżÄ¾Ģ棩µÄ±¬ÕØ·“Ó¦£¬¢ÜŹĒĒåĻ“ø½×ÅÓŚŹŌ¹ÜµČČŻĘ÷ÄŚ±ŚµÄ²ŠĮōĮņµÄ·½·ØÖ®Ņ»”£
“ÓĮņŌŚ·“Ó¦ÖŠµÄ¼ŪĢ¬±ä»Æ漲飬ĘäÖŠ________ÓėĘäӹȿøö²»Ķ¬£¬ĒėøųÓč¼ņŅŖµÄ·ÖĪö_______
_______________________________________________________________________________
 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
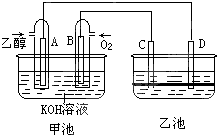 ČēĶ¼¼×³ŲŗĶŅŅ³ŲÖŠµÄĖÄøöµē¼«¶¼ŹĒ¶čŠŌ²ÄĮĻ£¬ŅŅ³ŲČÜŅŗ·Ö²ć£¬ÉĻ²ćČÜŅŗĪŖŃĪČÜŅŗ£¬³ŹÖŠŠŌ£¬Ēėøł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā£ŗ
ČēĶ¼¼×³ŲŗĶŅŅ³ŲÖŠµÄĖÄøöµē¼«¶¼ŹĒ¶čŠŌ²ÄĮĻ£¬ŅŅ³ŲČÜŅŗ·Ö²ć£¬ÉĻ²ćČÜŅŗĪŖŃĪČÜŅŗ£¬³ŹÖŠŠŌ£¬Ēėøł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| A”¢ÉĻŹöĖÄøö·“Ó¦¶¼ŹĒĪüČČ·“Ó¦ | ||
| B”¢1molŅŗĢ¬H2µÄÄÜĮæ“óÓŚ1molĘųĢ¬H2µÄÄÜĮæ | ||
| C”¢H2µÄČ¼ÉÕČČ£Ø”÷H£©ĪŖ-285.8kJ?mol-1 | ||
D”¢»š¼żÖŠŅŗĒāČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖH2£Øl£©+
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĪļĄķ½ĢŃŠŹŅ ĢāŠĶ£ŗ022
¢ŁS+HgØTØTHgS£¬¢Ś3S+2Al![]() Al2S3£¬
Al2S3£¬
¢ŪS+2KNO3+3C![]() K2S+N2”ü+3CO2”ü£¬
K2S+N2”ü+3CO2”ü£¬
¢Ü3S+6KOHØTØT2K2S+K2SO3+3H2O£¬ĘäÖŠ¢Ł³£ÓĆÓŚĻū³żŹŅÄŚ¹ÆÕōĘųµÄĪŪČ¾£¬¢ŚÓĆÓŚÖʱøAl2S3£¬¢ŪŹĒĪŅ¹ś¹Å“śĖÄ“ó·¢Ć÷Ö®Ņ»”Ŗ”ŖŗŚ»šŅ©£ØŅ»Įņ¶žĻõČżÄ¾Ģ棩µÄ±¬ÕØ·“Ó¦£¬¢ÜŹĒĒåĻ“ø½×ÅÓŚŹŌ¹ÜµČČŻĘ÷ÄŚ±ŚµÄ²ŠĮōĮņµÄ·½·ØÖ®Ņ»”£
“ÓĮņŌŚ·“Ó¦ÖŠµÄ¼ŪĢ¬±ä»Æ漲飬ĘäÖŠ________ÓėĘäӹȿøö²»Ķ¬£¬ĒėøųÓč¼ņŅŖµÄ·ÖĪö_______
_______________________________________________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĶ¬²½Ģā ĢāŠĶ£ŗµ„Ń”Ģā
[ ]
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com