


 דŌŖ·»Č«³ĢĶ»Ęʵ¼Į·²āĻµĮŠ“š°ø
דŌŖ·»Č«³ĢĶ»Ęʵ¼Į·²āĻµĮŠ“š°ø Ö±ĶعóÖŻĆūŠ£ÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
Ö±ĶعóÖŻĆūŠ£ÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®c(Al3+)=0.1mol/LµÄČÜŅŗÖŠ£ŗNa+”¢K+”¢AlO2-”¢OH- |
| B£®ĪŽÉ«ČÜŅŗÖŠ£ŗK+”¢CH3COO-”¢HCO3-”¢MnO4£ |
C£®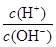 =1”Į1014µÄČÜŅŗ£ŗBa2+”¢Na+”¢SO32-”¢NO3- =1”Į1014µÄČÜŅŗ£ŗBa2+”¢Na+”¢SO32-”¢NO3- |
| D£®ĶØČė×ćĮæCO2ŗóĖłŗ¬Ąė×Ó»¹ÄÜ“óĮæ¹²“ęµÄŹĒ£ŗK+”¢Ca2+”¢NO3-”¢Cl- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Cl£”¢Na£«”¢OH£”¢Ba2£« | B£®Na£«”¢NO3£”¢SO42£”¢Mg2£« |
| C£®K£«”¢Na£«”¢Cl£”¢ ClO£ | D£®Ca2£«”¢NO3£”¢CO32£”¢Cl£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®c(OH-)=10-13mol”¤L-1µÄČÜŅŗÖŠ£ŗNa+”¢NH4+”¢Cl-”¢NO3- |
| B£®1.0 mol”¤L-1KIČÜŅŗÖŠ£ŗMg2+”¢Fe3+”¢SO42-”¢C1O- |
| C£®c(Fe3+)="0.1" mol”¤L-1µÄČÜŅŗÖŠ£ŗNa+”¢NH4+”¢HCO3-”¢SCN- |
| D£®±„ŗĶ°±Ė®ÖŠ£ŗNH4+”¢Ag+”¢SO42-”¢NO3- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ag(NH3)2£«”¢K+”¢Cl£”¢OH- | B£®S2-”¢Na£«”¢Cl£”¢ClO£ |
| C£®Al3£«”¢K+”¢SO32£”¢S2£ | D£®Fe3£«”¢Na£«”¢Cl£”¢SO42- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®pH£½1µÄČÜŅŗÖŠ£ŗNH4+”¢Fe2+”¢SO42-”¢Cl£ |
| B£®ĶØČė¹żĮæSO2ĘųĢåµÄČÜŅŗÖŠ£ŗFe3+”¢NO3£”¢Ba2+”¢H+ |
| C£®c(Al3+)£½0.1 mol/LµÄČÜŅŗÖŠ£ŗNa+”¢K+”¢AlO2£”¢SO42- |
| D£®ÓÉĖ®µēĄė³öµÄc(H+)£½1”Į10£13mol/LµÄČÜŅŗÖŠ£ŗNa+”¢HCO3£”¢Cl£”¢Br£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ļņ10mLpH=3µÄŗØĖįČÜŅŗÖŠ¼ÓČė10mLpH=l1µÄNaOHČÜŅŗ£¬»ģŗĻŅŗµÄpH=7 |
| B£®ŹµŃé²ā¶ØNH4HCO3ČÜŅŗĻŌ¼īŠŌ£¬CH3COONH4ČÜŅŗĻŌÖŠŠŌ£¬ĖµĆ÷ĖįŠŌCH3COOH>HCO3 |
| C£®NH4CIČÜŅŗ¼ÓĖ®Ļ”ŹĶ¹ż³ĢÖŠc£ØH+£©+c£ØNH3”¤H2O£©=c£ØOHŅ»£© |
| D£®ĻņAgI³ĮµķÖŠ¼ÓČĖ±„ŗĶKClČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬ĖµĆ÷AgCl±ČAgIøüÄŃČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ca2+”¢H+”¢HCO3-”¢C1- | B£®Ba2+”¢K+”¢OHŅ»”¢NO3- |
| C£®Cu2+”¢Na+”¢MnO4Ņ»”¢SO42Ņ» | D£®Fe3+”¢A13+”¢SCNŅ»”¢HCO3”Ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com