
ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1mol/L������ֵ����ˮ�ĵ��뼰���ӵ�ˮ�⣩��
| ������ | K+ Ag+ Mg2+ Cu2+ Al3+ |
| ������ | C1�� |
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��ȡ����ɫ��Һ5 mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӡ�
���ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���档
����ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
������������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
��1����I�����жϣ���Һ��һ�������е��������� ��
��2�����м�������������ɫ��������ӷ���ʽ�� ��
��3����ͬѧ����ȷ��ԭ��Һ�������������� ���������� �����ݴ��Ʋ�ԭ��ҺӦ�ó� �ԣ�ԭ���� ���������ӷ���ʽ˵������
��4����ȡ100 mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪ
����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ g��
��֪ʶ�㡿���������ӵļ��顢���������ӵļ���
���𰸽�������1��K+��NH��Cu2+��2�֣�
������2��6I��+2NO+8H+===3I2+2NO��+4H2O��2�֣�
������3��Mg2+��Al3+��2�֣�����Cl����NO��SO ��I����3�֣������ᣨ1�֣�
��I����3�֣������ᣨ1�֣�
�������� Mg2++2H2O Mg(OH)2+2H+��Al3++3H2O
Mg(OH)2+2H+��Al3++3H2O Al(OH)3+3H+��1�֣�д������һ�����ɣ�
Al(OH)3+3H+��1�֣�д������һ�����ɣ�
������4��Mg2++2OH��===Mg(OH)2����Al3++4OH��===AlO+2H2O��2�֣����������Ҳ�ɣ�����
�������� 0.4��1�֣�
��������ȡ����ɫ��Һ5mL��˵��һ��������Cu2+���μ�һ�ΰ�ˮ�г������ɣ��������������ӣ�˵�����ӵ���NH4+������ԭ��Һ��һ������NH4+�����ܺ���Mg2+��Al3+������NH4+��CO32-��
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ���棨����ɫ�ܲ����۲죩��˵��û��K+��
����ȡ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬˵���л�ԭ������ I-��NO3-��H+��Ӧ����NO������Һ����I-��NO3-�����ж�һ��������Ag+��
������������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɣ�˵����SO42-��
��1���ɢ��жϣ���Һ��һ�������е���������K+��NH4+��Cu2+��
��2�����м�����������������ɫ���壬��I-��NO3-��H+��Ӧ����NO�������ӷ���ʽ��6I-+2NO3-+8H+�T3I2+2NO��+4H2O��
��3��������������֪һ�����е�������I-��NO3-��SO42-���Ҹ�Ϊ0.1mol/L�����ݵ���غ��֪���ƶϳ��������Ӻ���Mg2+��Al3+����Ũ��Ϊ0.1mol/L���ɵ���غ��֪��Һ�л���һ��-1�۵�������ΪCl-�����Լ�ͬѧ����ȷ��ԭ��Һ�������������ǣ�Mg2+��Al3+���������ǣ�Cl-��I-��NO3-��SO42-����Һ��þ���Ӻ�������ˮ����Һ�����ԣ���Ӧ�����ӷ���ʽΪ��Mg2++2H2O⇌Mg��OH��2+2H+��Al3++3H2O⇌Al��OH��3+3H+��
��4����ȡ100mLԭ��Һ������������NaOH��Һ��Mg2+��Al3+ ��Ӧ����Mg��OH��2��NaAlO2���漰�����ӷ���ʽΪMg2++2OH-�TMg��OH��2����Al3++4OH-�TAlO2-+2H2O����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ�����Ԫ���غ㣺n��MgO��=n��Mg2+��=cV=0.1mol/L��0.1L=0.01mol��n��MgO��=0.01mol��40g/mol=0.4g��
��˼·�㲦�����⿼�����ʵļ��顢�����Լ�����ʽ���йؼ��㣬��Ŀ�Ѷ��еȣ������Ĺؼ��ǰ����йط�Ӧ�Ļ�ѧ����ʽ����д��


 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ij��Һϡ�����У���Һ�����ʵ���Ũ������Һ����ı仯����ͼ������ͼ�����ݷ����ó�aֵ���ڣ� ����
A��2 B��3 C��4 D��5
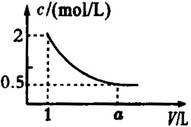
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʷ������ȷ����ǣ� ��
| �������� ��ϡ��� | �� | �� | �� | ���������� | ���������� |
| A | Na2CO3 | H2SO4 | NaHCO3 | SiO2 | CO2 |
| B | NaOH | HCl | NaCl | Na2O | CO |
| C | NaOH | CH3COOH | MgCl2 | CO2 | SO2 |
| D | KOH | HNO3 | CaCO3 | CaO | SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����״���µ�ij���壨Ħ������ΪM g ��mol��1������a��ˮ�У����õ�����Һ���ܶ�Ϊb g ��mL ��1�����ʵ���Ũ��Ϊc mol ��L��1 ��������ˮ�ĸ���������Ϊ �� ��
A��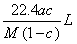 B��
B��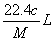 C��
C�� 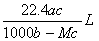 D��
D��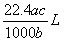
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA��ʾ�����ӵ�����������˵����ȷ���� ��������
A��30 g SiO2����NA��Si��O���ۼ�
B��1 L 0.2mol��L��1 Al2(SO4)3��Һ�е���������ΪNA
C����״���£�22.4 L H2O����ԭ�Ӹ�������3NA
D����4 mol HCl��Ũ���������MnO2���ȷ�Ӧ���Ƶ�Cl2�ķ�����ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ѧ����ѡ��5�л���ѧ��������15�֣�
G��һ�ֺϳ�����֬����Ҫԭ�ϣ�A��C��H��O����Ԫ����ɵ���Ԫ��״�������Է�������Ϊ98����˴Ź�������ֻ��һ���壻F�ĺ˴Ź���������3���壬�����֮��Ϊ2:2:3����֪��������R��������
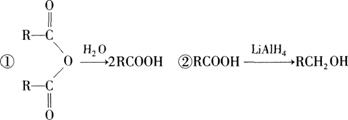
�й����ʵ�ת����ϵ����ͼ��ʾ����ش��������⡣
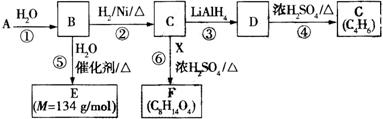
��1��A�в������Ĺ����ŵ������� ���ݵķ�Ӧ������ ��G�Ľṹ��ʽΪ ��G��Br2��CCl4��Һ��Ӧ�������� ��(�����������칹)��
��2����Ӧ�ڵĻ�ѧ����ʽΪ ��
��3��E����һ��������ͨ�� (�Ӧ����)���ɸ߷��ӻ����д���ø߷��ӻ�������ܵĽṹ��ʽ�� (���ּ���)��
��4����Ӧ�Ļ�ѧ����ʽΪ ��
��5���л���Y��E��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ������������Ŀ��д�����з���������Y�Ľṹ��ʽ�� ��Y����������������Һ��Ӧ���ò���֮һM���������ȵ�����ͭ��Ӧ��д��M�����ȵ�����ͭ��Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڿ��淴ӦH2(g)��I2(g) 2HI(g)����һ���¶�����H2(g)��I2(g)��ʼ��Ӧ������˵����ȷ����(����)
2HI(g)����һ���¶�����H2(g)��I2(g)��ʼ��Ӧ������˵����ȷ����(����)
A��H2(g)������������HI(g)����������֮��Ϊ2��1
B����Ӧ���еľ������������淴Ӧ����֮��
C�������淴Ӧ���ʵı�ֵ�Ǻ㶨��
D���ﵽƽ��ʱ��һ����c(H2) = c(HI)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Һ���ܴ��������һ�����ӻ������ �� ��
A��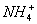 ��H����
��H����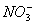 ��
��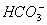
B��K+ ��Al3+��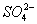 ��NH3��H2O
��NH3��H2O
C��Na+��K+��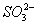 ��Cl2
��Cl2
D��Na+ ��CH3COO-��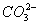 ��
��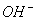
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.1 mol•.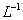 ������ζ�0.12 mol•.
������ζ�0.12 mol•.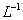 �İ�ˮ���ζ����������������ֵĽ����
�İ�ˮ���ζ����������������ֵĽ����
A.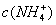 >
>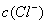 ,
,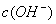 >
>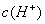 B.
B. 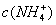 =
=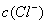 ,
,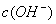 =
=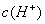
C.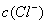 >
>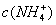 ,
,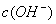 >
>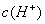 D.
D. 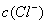 >
>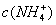 ,
,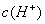 >
>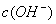
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com