
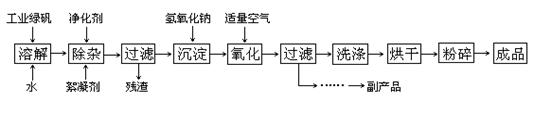
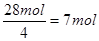 ������ͨ���Ŀ�����7mol��5��22.4L/mol��784L��
������ͨ���Ŀ�����7mol��5��22.4L/mol��784L��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���˷����ŵ�֮һ��ԭ��ȡ�Դ� |
| B�����Т٢ڢ۲����Ŀ���Ǹ���MgCl2 |
| C�����õ����������þ�ķ�����ȡþ |
| D����ⷨұ������Ҫ���Ĵ����ĵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ˮ���̺���Ԫ����80���֣�����Mg��Br��I�ں�ˮ�е��ܴ����ֱ�ԼΪ1.8��1015t��1��1014t��8��1010t�����ں�ˮ��þ�Ĵ����ܴ�ҵ�ϳ��Ժ�ˮΪԭ����ȡþ����ˣ�þԪ�ر���Ϊ������Ԫ�ء� |
B�����ڿ��淴ӦN2��g��+3H2��g��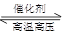 2NH3��g����������Ũ�ȿ����ӻ���Ӱٷ������Ӷ�ʹ��Ӧ�������� 2NH3��g����������Ũ�ȿ����ӻ���Ӱٷ������Ӷ�ʹ��Ӧ�������� |
| C�����Ṥҵ�У����������ϲ������������ٴ�ͨ��Ӵ��ҽ��ж�����������һ�����պ�������ĺ������٣���ֱ���ŷŵ������� |
| D���������ߺͦ�-����ɢ������ԭ�ӽṹģ�͵Ľ��������˹��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������ϳɰ����� | B���Ž��з���Ԫ�������� |
| C����Ӣ˹̹�������� | D��������˷����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���۲� | B����ѧ̽�� | C��˼�� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
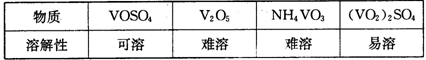
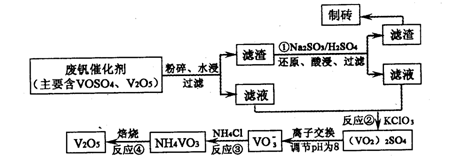
| A���ȷֽ�ʧȥH2O���ٷֽ�ʧȥNH3 | B���ȷֽ�ʧȥNH3���ٷֽ�ʧȥH2O |
| C��ͬʱ�ֽ�ʧȥH2O��NH3 | D��ͬʱ�ֽ�ʧȥH2��N2��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
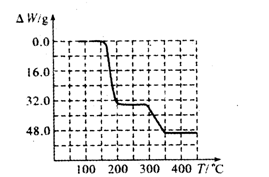
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ֻ����� |
| B����ѧ�������� |
| C��ʯӢ���� |
| D����ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���Ͽ�Ƭ | ||
| ���� | �۵�/�� | �е�/�� |
| SiCl4 | ��70 | 57.6 |
| TiCl4 | ��25 | 136.5 |
 SiO2)��̼�ۻ��װ���Ȼ�¯�У��ڸ�����ͨ��Cl2��Ӧ���Ƶû���SiCl4���ʵ�TiCl4��
SiO2)��̼�ۻ��װ���Ȼ�¯�У��ڸ�����ͨ��Cl2��Ӧ���Ƶû���SiCl4���ʵ�TiCl4�� �õ�����TiO2��xH2O��
�õ�����TiO2��xH2O��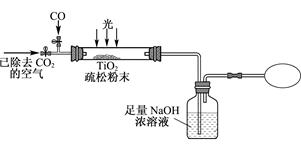
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com