
ŅŃÖŖNaHSO4ŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ĪŖNaHSO4===Na£«£«H£«£«SO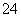 £¬Ä³ĪĀ¶ČĻĀ£¬ĻņpH£½6µÄÕōĮóĖ®ÖŠ¼ÓNaHSO4¾§Ģ壬±£³ÖĪĀ¶Č²»±ä£¬²āµĆČÜŅŗµÄpH£½2£¬¶ŌÓŚøĆČÜŅŗ£¬ĻĀĮŠŠšŹöÖŠ²»ÕżČ·µÄŹĒ £Ø””””£©
£¬Ä³ĪĀ¶ČĻĀ£¬ĻņpH£½6µÄÕōĮóĖ®ÖŠ¼ÓNaHSO4¾§Ģ壬±£³ÖĪĀ¶Č²»±ä£¬²āµĆČÜŅŗµÄpH£½2£¬¶ŌÓŚøĆČÜŅŗ£¬ĻĀĮŠŠšŹöÖŠ²»ÕżČ·µÄŹĒ £Ø””””£©
A£®øĆĪĀ¶ČøßÓŚ25”ę
B£®Ė®µēĄė³öµÄc£ØH£«£©£½1”Į10£10 mol/L
C£®c£ØH£«£©£½c£ØOH££©£«c£ØSO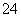 £©
£©
D£®øĆĪĀ¶ČĻĀ¼ÓČėµČĢå»żpH£½12µÄNaOHČÜŅŗæÉŹ¹·“Ó¦ŗóµÄČÜŅŗĒ”ŗĆ³ŹÖŠŠŌ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ (””””)”£
A£®½«AlCl3ČÜŅŗŗĶAl2(SO4)3ČÜŅŗ·Ö±š¼ÓČČ”¢ÕōøÉ”¢×ĘÉÕ£¬ĖłµĆ¹ĢĢå³É·ÖĻąĶ¬
B£®ÅäÖĘFeSO4ČÜŅŗŹ±£¬½«FeSO4¹ĢĢåČÜÓŚĻ”ŃĪĖįÖŠ£¬Č»ŗóĻ”ŹĶÖĮĖłŠčÅضČ
C£®ÓĆ¼ÓČȵķ½·ØæÉŅŌ³żČ„KClČÜŅŗÖŠµÄFe3£«
D£®Ļ“µÓÓĶĪŪ³£ÓĆČȵÄĢ¼ĖįÄĘČÜŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£®¹¤ŅµŗĻ³É°±ÓėÖʱøĻõĖįŅ»°ćæÉĮ¬ŠųÉś²ś£¬Į÷³ĢČēĶ¼ĖłŹ¾£ŗ
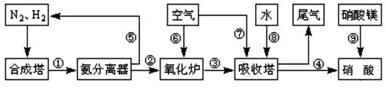
£Ø1£©¹¤ŅµÉś²śŹ±£¬ÖĘČ”ĒāĘųµÄŅ»øö·“Ó¦ĪŖ£ŗCO+H2O(g) CO2+H2
CO2+H2
T”ꏱ£¬Ķł1LĆܱÕČŻĘ÷ÖŠ³äČė0.2mol COŗĶ0.3molĖ®ÕōĘų”£·“Ó¦½ØĮ¢Ę½ŗāŗó£¬ĢåĻµÖŠc(H2)=0.12mol·L£1”£øĆĪĀ¶ČĻĀ“Ė·“Ó¦µÄĘ½ŗā³£ŹżK=_____£ØĢī¼ĘĖć½į¹ū£©”£
£Ø2£©ŗĻ³ÉĖžÖŠ·¢Éś·“Ó¦N2(g)+3H2(g) 2NH3(g)£»”÷H<0”£ĻĀ±ķĪŖ²»Ķ¬ĪĀ¶ČĻĀøĆ·“Ó¦µÄĘ½ŗā³£Źż”£ÓÉ“ĖæÉĶĘÖŖ£¬±ķÖŠT1____573K£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©”£
2NH3(g)£»”÷H<0”£ĻĀ±ķĪŖ²»Ķ¬ĪĀ¶ČĻĀøĆ·“Ó¦µÄĘ½ŗā³£Źż”£ÓÉ“ĖæÉĶĘÖŖ£¬±ķÖŠT1____573K£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©”£
| T/”ę | T1 | 300 | T2 |
| K | 1.00”Į107 | 2.45”Į105 | 1.88”Į103 |
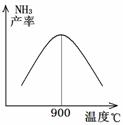
£Ø3£©N2ŗĶH2ŅŌĢś×÷“߻ƼĮ“Ó145”ę¾ĶæŖŹ¼·“Ó¦£¬²»Ķ¬ĪĀ¶ČĻĀNH3µÄ²śĀŹČēĶ¼ĖłŹ¾”£ĪĀ¶ČøßÓŚ900”ꏱ£¬NH3²śĀŹĻĀ½µµÄŌŅņŹĒ
£Ø4£©ĻõĖį³§µÄĪ²ĘųÖ±½ÓÅŷŽ«ĪŪČ¾æÕĘų£¬ÄæĒ°æĘѧ¼ŅĢ½Ė÷ĄūÓĆČ¼ĮĻĘųĢåÖŠµÄ¼×ĶéµČ½«µŖŃõ»ÆĪļ»¹ŌĪŖµŖĘųŗĶĖ®£¬Ęä·“Ó¦»śĄķĪŖ£ŗ
CH4(g)+4NO2=(g)£½4NO(g)+CO2(g)+2H2O(g)£» ”÷H=£574kJ·mol£1
CH4(g)+4NO(g)£½2N2(g)+CO2(g)+2H2O(g)£» ”÷H=£1160kJ·mol£1
Ōņ¼×ĶéÖ±½Ó½«NO2»¹ŌĪŖN2µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ
£Ø5£©°±ĘųŌŚ“æŃõÖŠČ¼ÉÕ£¬Éś³ÉŅ»ÖÖµ„ÖŹŗĶĖ®”£æĘѧ¼ŅĄūÓĆ“ĖŌĄķ£¬Éč¼Ę³É°±Ęų-ŃõĘųČ¼ĮĻµē³Ų£¬ŌņĶØČė°±ĘųµÄµē¼«¼īŠŌĢõ¼žĻĀ·¢Éś·“Ó¦µÄµē¼«·“Ó¦Ź½ĪŖ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
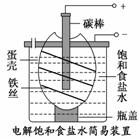
ČēĶ¼ĪŖµē½ā±„ŗĶŹ³ŃĪĖ®µÄ¼ņŅ××°ÖĆ£¬ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
A£®µē½āŅ»¶ĪŹ±¼äŗó£¬Ķłµ°æĒÖŠČÜŅŗÖŠµĪ¼Ó¼øµĪ·ÓĢŖ£¬³ŹŗģÉ«
B£®µ°æĒ±ķĆę²ųČʵÄĢśĖæ·¢ÉśŃõ»Æ·“Ó¦
C£®ĢśĖæ±ķĆęÉś³ÉµÄĘųĢåÄÜŹ¹ŹŖČóµÄµķ·Ūµā»Æ¼ŲŹŌÖ½±äĄ¶
D£®µ°æĒæÉ×čÖ¹Éś³ÉµÄĀČĘųÓėĒāĘų”¢ĒāŃõ»ÆÄĘČÜŅŗ½Ó“„
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
LiFePO4ŠĀŠĶļ®Ąė×Ó¶ÆĮ¦µē³ŲŅŌĘ䶥ĢŲµÄÓÅŹĘ³ÉĪŖ°ĀŌĖ»įĀĢÉ«ÄÜŌ“µÄŠĀ³č£®ŅŃÖŖøƵē³Ų·ÅµēŹ±µÄµē¼«·“Ó¦Ź½ĪŖ£ŗÕż¼«FePO4£«Li£«£«e£===LiFePO4£¬øŗ¼«Li£e£===Li£«£®ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ
£Ø””””£©
A£®³äµēŹ±µÄ×Ü·“Ó¦ĪŖFePO4£«Li===LiFePO4
B£®³äµēŹ±¶ÆĮ¦µē³ŲÉĻ±ź×¢”°£«”±µÄµē¼«Ó¦ÓėĶā½ÓµēŌ“µÄÕż¼«ĻąĮ¬
C£®·ÅµēŹ±µē³ŲÄŚ²æLi£«Ļņøŗ¼«ŅʶÆ
D£®·ÅµēŹ±£¬ŌŚÕż¼«ÉĻŹĒLi£«µĆµē×Ó±»»¹Ō
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijĖįHXĻ”ČÜŅŗŗĶij¼īYOHĻ”ČÜŅŗµÄĪļÖŹµÄĮæÅضČĻąµČ£¬Į½ČÜŅŗ»ģŗĻŗó£¬ČÜŅŗµÄpH“óÓŚ7£¬ĻĀ±ķÖŠÅŠ¶ĻŗĻĄķµÄŹĒ £Ø””””£©
| ±ąŗÅ | HX | YOH | ČÜŅŗµÄĢå»ż¹ŲĻµ |
| ¢Ł | ĒæĖį | Ēæ¼ī | V£ØHX£©£½V£ØYOH£© |
| ¢Ś | ĒæĖį | Ēæ¼ī | V£ØHX£©£¼V£ØYOH£© |
| ¢Ū | ĒæĖį | Čõ¼ī | V£ØHX£©£½V£ØYOH£© |
| ¢Ü | ČõĖį | Ēæ¼ī | V£ØHX£©£½V£ØYOH£© |
A£®¢Ł¢Ū B£®¢Ś¢Ū
C£®¢Ł¢Ü D£®¢Ś¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ī“Ą“ŠĀÄÜŌ“µÄĢŲµćŹĒ׏Ō“·įø»£¬ŌŚŹ¹ÓĆŹ±¶Ō»·¾³ĪŽĪŪČ¾»ņĪŪČ¾ŗÜŠ”£¬ĒŅæÉŅŌŌŁÉś£®ĻĀĮŠŹōÓŚĪ“Ą“ŠĀÄÜŌ“±ź×¼µÄŹĒ£Ø””””£©
¢ŁĢģČ»Ęų ¢ŚĆŗ ¢ŪŹÆÓĶ ¢ÜĢ«ŃōÄÜ ¢ŻÉśĪļÖŹÄÜ ¢Ž·ēÄÜ ¢ßĒāÄÜ£®
| ”” | A£® | ¢Ł¢Ś¢Ū¢Ü | B£® | ¢Ü¢Ż¢Ž¢ß | C£® | ¢Ū¢Ż¢Ž¢ß | D£® | ¢Ū¢Ü¢Ż¢Ž¢ß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¶ŌÓŚČĪŗĪŅ»øö»ÆŃ§Ę½ŗāĢåĻµ£¬²ÉČ”ŅŌĻĀ“ėŹ©£¬Ņ»¶Ø»įŹ¹Ę½ŗā·¢ÉśŅĘ¶ÆµÄŹĒ£Ø””””£©
| ”” | A£® | Ź¹ÓĆ“ß»Æ¼Į | B£® | ÉżøßĪĀ¶Č |
| ”” | C£® | Ōö“óĢåĻµŃ¹Ēæ | D£® | ¼ÓČėŅ»ÖÖ·“Ó¦Īļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀ±ķŹĒ²»Ķ¬ĪĀ¶ČĻĀĖ®µÄĄė×Ó»ż³£Źż£ŗ
| ĪĀ¶Č/”ę | 25 | t1 | t2 |
| Ė®µÄĄė×Ó»ż³£Źż | 1”Į10﹣14 | KW | 1”Į10﹣12 |
ŹŌ»Ų“šŅŌĻĀĪŹĢā£ŗ
£Ø1£©Čō25”ę£¼t1£¼t2£¬ŌņKW””””1”Į10﹣14£ØĢī”°£¾”±”¢”°£¼”±»ņ”°=”±£©£¬ÅŠ¶ĻµÄĄķÓÉŹĒ””””
£Ø2£©25”ęĻĀ£¬½«pH=13µÄĒāŃõ»ÆÄĘČÜŅŗV1 LÓėpH=1µÄĻ”ŃĪĖįV2 L»ģŗĻ£ØÉč»ģŗĻŗóČÜŅŗµÄĢå»żĪŖ£ŗV1+V2£©£¬ĖłµĆ»ģŗĻČÜŅŗµÄpH=2£¬ŌņV1£ŗV2=””””£®
£Ø3£©½«µČĢå»żµČĪļÖŹµÄĮæÅØ¶ČµÄ“×ĖįŗĶĒāŃõ»ÆÄĘČÜŅŗ»ģŗĻŗó£¬ČÜŅŗ³Ź””””£ØĢī”°Ėį
ŠŌ”±”¢”°ÖŠŠŌ”±»ņ”°¼īŠŌ”±£©£¬ČÜŅŗÖŠc£ØNa+£©””””c£ØCH3COO﹣£©£ØĢī”°£¾”±”¢”°=”±»ņ”°£¼”±£©£®
£Ø4£©pH=3µÄ“×ĖįŗĶpH=11µÄĒāŃõ»ÆÄĘČÜŅŗµČĢå»ż»ģŗĻŗóČÜŅŗ³Ź””””ŠŌ£ØĢī”°Ėį”±”¢”°ÖŠ”±
»ņ”°¼ī”±£©£¬ČÜŅŗÖŠc£ØNa+£©””””c£ØCH3COO﹣£©[Ģī”°£¾”±”¢”°=”±»ņ”°£¼”±]
£Ø5£©ŅŃÖŖNH4AČÜŅŗĪŖÖŠŠŌ£¬ÓÖÖŖHAČÜŅŗ¼Óµ½Na2CO3ČÜŅŗÖŠÓŠĘųĢå·Å³ö£¬ŹŌĶʶĻ£ØNH4£©2CO3ČÜŅŗµÄ
pH””””7£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©£»½«Ķ¬ĪĀ¶ČĻĀµČÅØ¶ČµÄĖÄÖÖŃĪČÜŅŗ£ŗ
A£®NH4HCO3 B£®NH4A C£®£ØNH4£©2SO4 D£®NH4Cl
°“pHÓɓ󵽊”µÄĖ³ŠņÅÅĮŠŹĒ£ŗ””””£ØĢīŠņŗÅ£©£®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com