
(7��)����ƽ�����ʢ��ǿ��ԭ��Һ̬��(N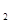 H
H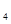 ��)��ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȡ���֪0.4 molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256 KJ��������
��)��ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȡ���֪0.4 molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256 KJ��������
��1����Ӧ���Ȼ�ѧ����ʽΪ ��
(2)����֪H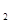 0(I)=H
0(I)=H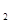 0(g)����H=+44 kJ��mol����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������
KJ��
0(g)����H=+44 kJ��mol����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������
KJ��
(3)�˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��� ��
(4)��֪N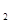 (g)+2O
(g)+2O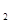 (g)=2N0
(g)=2N0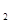 (g)
��H=+67.7kJ��mol
(g)
��H=+67.7kJ��mol
N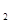 H
H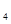 (g)+0
(g)+0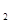 (g)=N
(g)=N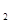 (g)+2H
(g)+2H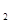 0(g)
��H=-534kJ��mol
0(g)
��H=-534kJ��mol
������N0 ��ȫ��Ӧ���ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽΪ
��ȫ��Ӧ���ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽΪ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� 6 �֣��ش��������⣺
��1����ӦA(g)+B(g)![]() C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)=CO (g)+ H2O (g)�� ��H1= + 34.0 kJ/mol
����(g)=CO2 (g)+ H2(g) ��H2=��7.0 kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2 ����̬H2������̬CO����̬H2O���Ȼ�ѧ����ʽΪ ��
��3������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ��H2O2����
����0.4molҺ̬�º�0.8molҺ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ
������(�൱��25�桢101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ����һ�и߶�ģ���⻯ѧ�Ծ� ���ͣ������
�� 6 �֣��ش��������⣺
��1����ӦA(g)+B(g) C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0 ���������������������ȷ��������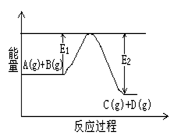
��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)=" CO" (g)+ H2O (g)�� ��H1=" +" 34.0 kJ/mol
����(g)= CO2 (g)+ H2(g) ��H2= ��7.0 kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2����̬H2������̬CO����̬H2 O���Ȼ�ѧ����ʽΪ ��
O���Ȼ�ѧ����ʽΪ ��
��3������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ�� ����Һ̬˫��ˮ��H2O2����
����Һ̬˫��ˮ��H2O2����
����0.4molҺ̬�º�0.8mol Һ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ
������(�൱��25�桢101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(7��)����ƽ�����ʢ��ǿ��ԭ��Һ̬��(N![]() H
H![]() ��)��ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȡ���֪0.4 molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256 KJ��������
��)��ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȡ���֪0.4 molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256 KJ��������
��1����Ӧ���Ȼ�ѧ����ʽΪ ��
(2)����֪H 0(I)=H
0(I)=H 0(g)����H=+44 kJ��mol����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų��������� KJ��
0(g)����H=+44 kJ��mol����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų��������� KJ��
(3)�˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��� ��
(4)��֪N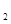 (g)+2O
(g)+2O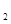 (g)=2N0
(g)=2N0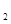 (g) ��H=+67.7kJ��mol
(g) ��H=+67.7kJ��mol
N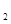 H
H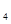 (g)+0
(g)+0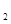 (g)=N
(g)=N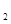 (g)+2H
(g)+2H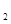 0(g) ��H=-534kJ��mol
0(g) ��H=-534kJ��mol
������N0![]() ��ȫ��Ӧ���ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽΪ
��ȫ��Ӧ���ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ����и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(7��)����ƽ�����ʢ��ǿ��ԭ��Һ̬��(N H
H ��)��ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȡ���֪0.4 molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256 KJ��������
��)��ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȡ���֪0.4 molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256 KJ��������
��1����Ӧ���Ȼ�ѧ����ʽΪ ��
(2)����֪H 0(I)=H
0(I)=H 0(g)����H="+44" kJ��mol����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų��������� KJ��
0(g)����H="+44" kJ��mol����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų��������� KJ��
(3)�˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��� ��
(4)��֪N (g)+2O
(g)+2O (g)=2N0
(g)=2N0 (g) ��H=+67.7kJ��mol
(g) ��H=+67.7kJ��mol
N H
H (g)+0
(g)+0 (
( g)=N
g)=N (g)+2H
(g)+2H 0(g) ��H=-534kJ��mol
0(g) ��H=-534kJ��mol
������N0 ��ȫ��Ӧ���ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽΪ
��ȫ��Ӧ���ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽΪ 
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com