
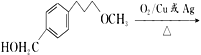
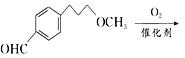
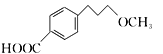
”¾ĢāÄæ”æĆĄĶŠĀå¶ūŹĒŅ»ÖÖÖĪĮĘøßŃŖŃ¹µÄŅ©ĪļµÄÖŠ¼äĢ壬æÉŅŌĶعżŅŌĻĀ·½·ØŗĻ³É£ŗ
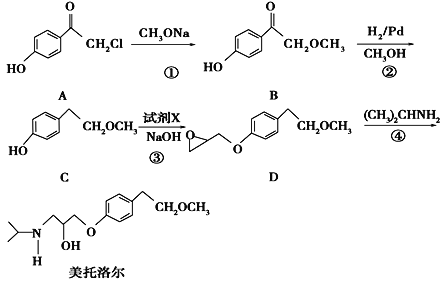
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öCÖŠµÄ¹ŁÄÜĶŵÄĆū³ĘĪŖ_________________.
£Ø2£©ĆĄĶŠĀå¶ūµÄ·Ö×ÓŹ½________________.
£Ø3£©Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½__________________________________________£»·“Ó¦¢ŚµÄ·“Ó¦ĄąŠĶŹĒ_____________
£Ø4£©·“Ó¦¢ŪÖŠ¼ÓČėµÄŹŌ¼ĮXµÄ·Ö×ÓŹ½ĪŖC3H5OCl£¬XµÄ½į¹¹¼ņŹ½ĪŖ____________________.
£Ø5£©Āś×ćĻĀĮŠĢõ¼žµÄBµÄĶ¬·ÖŅģ¹¹ĢåÓŠÓŠ_______ÖÖ£¬ĘäÖŠŗĖ“Ź²ÕńĒāĘ×ÓŠĮłÖÖ²»Ķ¬»Æѧ»·¾³µÄĒā£¬ĒŅ·åĆ껿±ČĪŖ3”Ć2”Ć2”Ć1”Ć1”Ć1µÄŹĒ________________________(Š“½į¹¹¼ņŹ½)
¢ŁÄÜ·¢ÉśŅų¾µ·“Ó¦¶ųĒŅÄÜ·¢ÉśĖ®½ā
¢ŚÄÜÓėFeCl3ČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦
¢ŪÖ»ÓŠŅ»øö¼×»ł
£Ø6£©øł¾ŻŅŃÓŠÖŖŹ¶²¢½įŗĻĢāÄæĖłøųĻą¹ŲŠÅĻ¢£¬Š“³öŅŌ![]() ŗĶ
ŗĶ ĪŖŌĮĻÖʱø
ĪŖŌĮĻÖʱø![]() µÄŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼(ĪŽ»śŹŌ¼ĮČĪŃ”)”£ŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ź¾ĄżČēĻĀ£ŗ
µÄŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼(ĪŽ»śŹŌ¼ĮČĪŃ”)”£ŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ź¾ĄżČēĻĀ£ŗ![]() _______________________
_______________________
”¾“š°ø”æ ōĒ»ł””ĆŃ¼ü C15H25O3N  +CH3ONa”ś
+CH3ONa”ś![]() + NaCl »¹Ō·“Ó¦
+ NaCl »¹Ō·“Ó¦ ![]() 23
23 
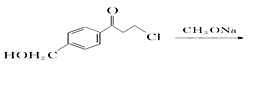
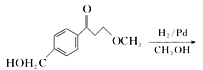
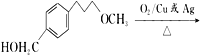
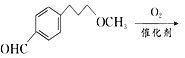
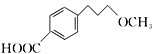
”¾½āĪö”ææ¼²éÓŠ»śĪļµÄĶʶĻŗĶŗĻ³É£¬£Ø1£©øł¾ŻCµÄ½į¹¹¼ņŹ½£¬ŗ¬ÓŠ¹ŁÄÜĶÅŹĒōĒ»łŗĶĆŃ¼ü£»£Ø2£©øł¾ŻÓŠ»śĪļ³É¼üĢŲµć£¬ĆĄĶŠĀå¶ūµÄ·Ö×ÓŹ½ĪŖC15H25O3N£»£Ø3£©øł¾ŻAŗĶB½į¹¹¼ņŹ½µÄ¶Ō±Č£¬·“Ó¦¢ŁĪŖČ”“ś·“Ó¦£¬Ęä·“Ó¦·½³ĢŹ½ĪŖ  +CH3ONa”ś
+CH3ONa”ś![]() + NaCl£»øł¾ŻBŗĶC½į¹¹¼ņŹ½µÄ¶Ō±Č£¬BÖŠōŹ»łÉĻµÄŃõŌ×Ó×Ŗ»Æ³ÉHŌ×Ó£¬“Ė·“Ó¦ĪŖ»¹Ō·“Ó¦£»£Ø4£©¶Ō±ČCŗĶD½į¹¹¼ņŹ½£¬·“Ó¦¢Ū·¢ÉśµÄČ”“ś·“Ó¦£¬¼“ŹŌ¼ĮX½į¹¹¼ņŹ½ĪŖ£ŗ
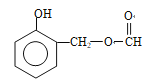
+ NaCl£»øł¾ŻBŗĶC½į¹¹¼ņŹ½µÄ¶Ō±Č£¬BÖŠōŹ»łÉĻµÄŃõŌ×Ó×Ŗ»Æ³ÉHŌ×Ó£¬“Ė·“Ó¦ĪŖ»¹Ō·“Ó¦£»£Ø4£©¶Ō±ČCŗĶD½į¹¹¼ņŹ½£¬·“Ó¦¢Ū·¢ÉśµÄČ”“ś·“Ó¦£¬¼“ŹŌ¼ĮX½į¹¹¼ņŹ½ĪŖ£ŗ![]() £»£Ø5£©ÄÜ·¢ÉśŅų¾µ·“Ó¦ĒŅÄÜ·¢ÉśĖ®½ā£¬ĖµĆ÷“ĖĪļÖŹÓ¦ŹĒ¼×Ėįijõ„£¬ÄÜÓėFeCl3ČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦£¬ĖµĆ÷ŗ¬ÓŠ·ÓōĒ»ł£¬·ūŗĻĢõ¼žµÄĶ¬·ÖŅģ¹¹Ģå£ŗ
£»£Ø5£©ÄÜ·¢ÉśŅų¾µ·“Ó¦ĒŅÄÜ·¢ÉśĖ®½ā£¬ĖµĆ÷“ĖĪļÖŹÓ¦ŹĒ¼×Ėįijõ„£¬ÄÜÓėFeCl3ČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦£¬ĖµĆ÷ŗ¬ÓŠ·ÓōĒ»ł£¬·ūŗĻĢõ¼žµÄĶ¬·ÖŅģ¹¹Ģå£ŗ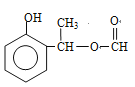 ”¢
”¢ £Ø¼×»łŌŚ±½»·ÉĻĪ»ÖĆÓŠ4ÖÖ£©”¢
£Ø¼×»łŌŚ±½»·ÉĻĪ»ÖĆÓŠ4ÖÖ£©”¢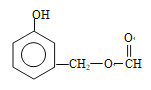 (¼×»łŌŚ±½»·ÉĻĪ»ÖĆÓŠ4ÖÖ)”¢
(¼×»łŌŚ±½»·ÉĻĪ»ÖĆÓŠ4ÖÖ)”¢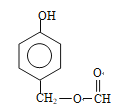 £Ø¼×»łŌŚ±½»·ÉĻĪ»ÖĆÓŠ2ÖÖ£©”¢
£Ø¼×»łŌŚ±½»·ÉĻĪ»ÖĆÓŠ2ÖÖ£©”¢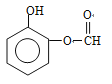 £ØŅŅ»łŌŚ±½»·ÉĻĪ»ÖĆÓŠ4ÖÖ£©”¢
£ØŅŅ»łŌŚ±½»·ÉĻĪ»ÖĆÓŠ4ÖÖ£©”¢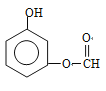 £ØŅŅ»łŌŚ±½»·ÉĻĪ»ÖĆÓŠ4ÖÖ£©”¢
£ØŅŅ»łŌŚ±½»·ÉĻĪ»ÖĆÓŠ4ÖÖ£©”¢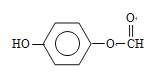 £ØŅŅ»łŌŚ±½»·ÉĻµÄĪ»ÖĆÓŠ2ÖÖ£©£¬¹²ÓŠ23ÖÖ£¬ÓŠĮłÖÖ²»Ķ¬µÄ»Æѧ»·¾³µÄĒā£¬ĒŅ·åĆ껿±ČĪŖ3£ŗ2£ŗ2£ŗ1£ŗ1£ŗ1£¬Ņņ“Ė½į¹¹¼ņŹ½ĪŖ£ŗ
£ØŅŅ»łŌŚ±½»·ÉĻµÄĪ»ÖĆÓŠ2ÖÖ£©£¬¹²ÓŠ23ÖÖ£¬ÓŠĮłÖÖ²»Ķ¬µÄ»Æѧ»·¾³µÄĒā£¬ĒŅ·åĆ껿±ČĪŖ3£ŗ2£ŗ2£ŗ1£ŗ1£ŗ1£¬Ņņ“Ė½į¹¹¼ņŹ½ĪŖ£ŗ £»£Ø6£©øł¾ŻĆĄĶŠĀå¶ūŗĻ³ÉĀ·Ļߣ¬µĆ³ö£ŗ
£»£Ø6£©øł¾ŻĆĄĶŠĀå¶ūŗĻ³ÉĀ·Ļߣ¬µĆ³ö£ŗ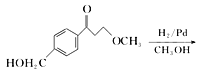
 ӣ
ӣ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓĆ¼×“¼Č¼ĮĻµē³Ų×÷µēŌ“£¬ÓĆĢś×÷µē¼«µē½āŗ¬Cr2O72-µÄĖįŠŌ·ĻĖ®£¬ĄūÓĆÉś³ÉµÄFe2+æɽ«Cr2O72-×Ŗ»Æ³ÉCr(OH)3³Įµķ¶ų³żČ„£¬×°ÖĆČēĶ¼”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
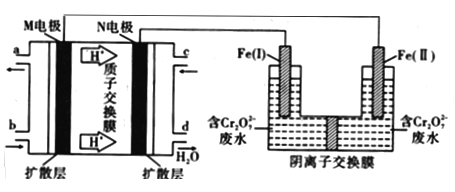
A. ÓÉbæŚ¼ÓČėĪļÖŹĪŖO2
B. Č¼ĮĻµē³Ųøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖCH3OH+8OH--6e-= CO32-+6H2O
C. µē½ā¹ż³ĢÖŠ£¬Fe(I)ÖŹĮæ¼õÉŁ£¬Fe(¢ņ)ÉĻÓŠĘųĢå²śÉś
D. µēĀ·ÖŠĆæ×ŖŅĘ6molµē×Ó£¬×ī¶ąÓŠ1mol Cr2O72-±»»¹Ō
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©1.5 molŃõĘųÖŠŗ¬ÓŠµÄO2µÄøöŹżĪŖ_____________£¬±ź×¼×“æöĻĀĢå»żĪŖ_________L£¬Óė_____________gH2OĖłŗ¬µÄŌ×Ó×ÜŹżĻąµČ”£
£Ø2£©ŌŚ±ź×¼×“æöĻĀ£¬Ģå»żĪŖ6.72LµÄNOŗĶNO2»ģŗĻĘų£¬ÖŹĮæĪŖ11.88g£¬ŌņNOŗĶNO2µÄĢå»ż±ČĪŖ___________”£
£Ø3£©Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬Į½ÖÖĘųĢåAŗĶBµÄĢå»żÖ®±ČĪŖ2”Ć1£¬ÖŹĮæÖ®±ČĪŖ8”Ć5£¬ŌņAÓėBµÄĆܶČÖ®±ČĪŖ________£¬Ä¦¶ūÖŹĮæÖ®±ČĪŖ_______”£
£Ø4£©Ä³×“æöĻĀ£¬2g¶žŃõ»ÆĢ¼ĘųĢåµÄĢå»żŹĒ1120 mL£¬2gAĘųĢåµÄĢå»żŹĒ770 mL£¬AµÄĦ¶ūÖŹĮæŹĒ______________”£
£Ø5£©Ä³ĮņĖįÄĘČÜŅŗÖŠŗ¬ÓŠ3.01”Į1022øöNa+£¬ŌņČÜŅŗÖŠSO42£µÄĪļÖŹµÄĮæŹĒ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĀĮ¼°Ęä»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²ś¼°ČÕ³£Éś»īÖŠÓŠÖŲŅŖÓĆĶ¾”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AlŌ×ӵļŪµē×ÓÅŲ¼Ķ¼ĪŖ_________________________________£¬Na”¢Mg”¢AlµÄµŚŅ»µēĄėÄÜÓÉŠ”µ½“óµÄĖ³ŠņĪŖ________________________”£
£Ø2£©Ä³ŗ¬ÓŠĀĮŌŖĖŲµÄōä“äµÄ»ÆѧŹ½ĪŖBe3Al2(Si6O18)£¬ĘäÖŠSiŌ×ÓµÄŌӻƹģµĄĄąŠĶĪŖ________”£
£Ø3£©¹¤ŅµÉĻÓĆŃõ»ÆĀĮ”¢µŖĘų”¢Ģ¼µ„ÖŹŌŚøßĪĀĢõ¼žĻĀæÉÖʱøŅ»ÖÖĖÄĆęĢå½į¹¹µ„ŌŖµÄøßĪĀ½į¹¹ĢÕ“É£¬Ę侧°ūČēĶ¼ĖłŹ¾£ŗ
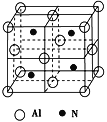
¢ŁøĆÖʱø·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ___________________________________.
¢ŚøĆ»ÆŗĻĪļµÄ¾§ĢåĄąŠĶĪŖ_______________£¬øĆ¾§°ūÖŠÓŠ____øöĀĮŌ×Ó£¬øĆ¾§°ūµÄ±ß³¤ĪŖa pm£¬ŌņøĆ¾§°ūµÄĆܶČĪŖ____________g”¤cm£3”£
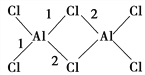
£Ø4£©AlCl3µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ133.5£¬183 ”ęæŖŹ¼Éż»Ŗ£¬Ņ×ČÜÓŚĖ®”¢ŅŅĆŃµČ£¬Ę䶞¾ŪĪļ(Al2Cl6)µÄ½į¹¹ČēĶ¼ĖłŹ¾£¬Ķ¼ÖŠ1¼ü¼ü³¤ĪŖ206 pm,2¼ü¼ü³¤ĪŖ221 pm£¬“Ó¼üµÄŠĪ³É½Ē¶Č·ÖĪö1¼üŗĶ2¼üµÄĒų±š£ŗ__________________________________________”£
£Ø5£©LiAlH4ŹĒŅ»ÖÖĢŲŹāµÄ»¹Ō¼Į£¬æɽ«ōČĖįÖ±½Ó»¹Ō³É“¼£ŗ
CH3COOH ![]() CH3CH2OH
CH3CH2OH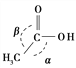
CH3COOH·Ö×ÓÖŠ¦Š¼üŗĶ¦Ņ¼üµÄŹżÄæÖ®±ČĪŖ________£¬·Ö×ÓÖŠ¼ü½Ē¦Į________¼ü½Ē¦Ā(Ģī”°“óÓŚ”±”¢”°µČÓŚ”±»ņ”°Š”ÓŚ”±)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”涞ĆÆĢśæÉÓĆ×÷Č¼ĮĻµÄ½ŚÄÜĻūŃĢ¼Į”¢æ¹±¬¼ĮµČ”£ŹµŃéŹŅÖʱø¶žĆÆĢś×°ÖĆŹ¾ŅāĶ¼ČēĶ¼Ņ»
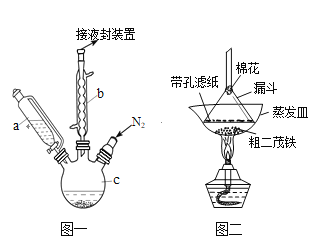
ŅŃÖŖ£ŗ¢Ł¶žĆÆĢśČŪµćŹĒ173”ę£¬ŌŚ100”ꏱæŖŹ¼Éż»Ŗ£»·ŠµćŹĒ249”ę”£
¢ŚÖʱø¶žĆÆĢśµÄ·“Ó¦ŌĄķŹĒ£ŗ2KOH+FeCl2+2C5H6= Fe(C5H5)2+2KCl+2H2O
ŹµŃé²½ÖčĪŖ£ŗ
¢ŁŌŚČż¾±ÉÕĘæÖŠ¼ÓČė25g·ŪĩדµÄKOH£¬²¢“ÓŅĒĘ÷aÖŠ¼ÓČė60mLĪŽĖ®ŅŅĆѵ½ÉÕĘæÖŠ£¬³ä·Ö½Į°č£¬Ķ¬Ź±ĶصŖĘųŌ¼10min£»
¢ŚŌŁ“ÓŅĒĘ÷aµĪČė5.5mLŠĀÕōĮóµÄ»·Īģ¶žĻ©£ØC5H6, ĆܶČĪŖ0.95g/cm3£©£¬½Į°č£»
¢Ū½«6.5gĪŽĖ®FeCl2Óė(CH3)2SO£Ø¶ž¼×ŃĒķ棬×÷ČܼĮ£©Åä³ÉµÄČÜŅŗ25mL×°ČėŅĒĘ÷aÖŠ£¬ĀżĀżµĪČėŅĒĘ÷cÖŠ£¬45minµĪĶź£¬¼ĢŠų½Į°č45min£»
¢ÜŌŁ“ÓŅĒĘ÷a¼ÓČė25mLĪŽĖ®ŅŅĆŃ½Į°č£»
¢Ż½«cÖŠµÄŅŗĢå×ŖČė·ÖŅŗĀ©¶·ÖŠ£¬ŅĄ“ĪÓĆŃĪĖį”¢Ė®ø÷Ļ“µÓĮ½“Ī£¬·ÖŅŗµĆ³Č»ĘÉ«ČÜŅŗ£»
¢ŽÕō·¢³Č»ĘÉ«ČÜŅŗ£¬µĆ¶žĆÆĢś“Ö²śĘ·”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŅĒĘ÷bµÄĆū³ĘŹĒ________________________.
£Ø2£©²½Öč¢ŁÖŠĶØČėµŖĘųµÄÄæµÄŹĒ____________________________________________________.
£Ø3£©ŅĒĘ÷cµÄŹŹŅĖČŻ»żÓ¦ĪŖ(Ń”±ąŗÅ)£ŗ_________
¢Ł100mL ¢Ś250mL ¢Ū500mL
£Ø4£©²½Öč¢ŻÓĆŃĪĖįĻ“µÓµÄÄæµÄŹĒ__________________________________________________
£Ø5£©²½Öč¢ßŹĒ¶žĆÆĢś“Ö²śĘ·µÄĢį“棬øĆ¹ż³ĢŌŚĶ¼¶žÖŠ½ųŠŠ£¬Ęä²Ł×÷Ćū³ĘĪŖ_________£»øĆ²Ł×÷ÖŠĆŽ»ØµÄ×÷ÓĆŹĒ______________________________________________________.
£Ø6£©ĪŖĮĖČ·ČĻµĆµ½µÄŹĒ¶žĆÆĢś£¬»¹ŠčŅŖ½ųŠŠµÄŅ»Ļī¼ņµ„ŹµŃéŹĒ__________________________£»Čō×īÖÕÖĘµĆ“æ¾»µÄ¶žĆÆĢś4.3g£¬ŌņøĆŹµŃéµÄ²śĀŹĪŖ____________£Ø±£ĮōČżĪ»ÓŠŠ§Źż×Ö£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŗ¬Ā±×åŌŖĖŲµÄ»ÆŗĻĪļŌŚæĘŃŠŗĶÉś²śÖŠÓŠŠķ¶ąÖŲŅŖÓĆĶ¾”£Čē£ŗ2molSb(CH3)3”¢2mol Sb (CH3)2BrŗĶ2 molSb(CH3)Br2½ųŠŠÖŲ×é·“Ó¦æÉÉś³ÉæÕ¼äĪ»×č×īŠ”µÄĄė×Ó»ÆŗĻĪļ[Sb2(CH3)5]2[Sb2(CH3)2Br6]”£Ēė»Ų“š£ŗ
£Ø1£©  ÖŠ£¬H”¢C”¢BrµÄµēøŗŠŌÓɓ󵽊”µÄĖ³ŠņĪŖ________£¬äåŌ×ÓµÄMÄܲćµē×ÓÅŲ¼Ź½ĪŖ________”£
ÖŠ£¬H”¢C”¢BrµÄµēøŗŠŌÓɓ󵽊”µÄĖ³ŠņĪŖ________£¬äåŌ×ÓµÄMÄܲćµē×ÓÅŲ¼Ź½ĪŖ________”£
£Ø2£©[Sb2(CH3)5]+µÄ½į¹¹Ź½ĪŖ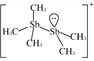 £¬SbŌ×ÓµÄŌӻƹģµĄĄąŠĶĪŖ_________”£Š“³öŅ»ÖÖÓėCH
£¬SbŌ×ÓµÄŌӻƹģµĄĄąŠĶĪŖ_________”£Š“³öŅ»ÖÖÓėCH![]() »„ĪŖµČµē×ÓĢåµÄŃōĄė×Ó_________”£
»„ĪŖµČµē×ÓĢåµÄŃōĄė×Ó_________”£
£Ø3£©µāŌŚĖ®ÖŠµÄČܽā¶ČĖäČ»Š”£¬µ«ŌŚµā»Æ¼ŲČÜŅŗÖŠČܽā¶ČČ“Ć÷ĻŌŌö“ó£¬ÕāŹĒÓÉÓŚ·¢ÉśI-+I2![]() I
I![]() ӣI
”£I![]() Ąė×ÓµÄæռ乹ŠĶĪŖ__________”£
Ąė×ÓµÄæռ乹ŠĶĪŖ__________”£
£Ø4£©Ņ»¶ØĢõ¼žĻĀSbCl3ÓėGaCl3ŅŌĪļÖŹµÄĮæÖ®±ČĪŖl£ŗl»ģŗĻµĆµ½Ņ»ÖÖ¹ĢĢ¬Ąė×Ó»ÆŗĻĪļ£¬Ęä½į¹¹×é³ÉæÉÄÜĪŖ£ŗ(a)[SbCl![]() ][GaCl
][GaCl![]() ]»ņ(b)[GaCl
]»ņ(b)[GaCl![]() ][SbCl
][SbCl![]() ]£¬øĆĄė×Ó»ÆŗĻĪļ×īæÉÄܵĽį¹¹×é³ÉĪŖ________ (Ģī”°a”±»ņ”°b”±)£¬ĄķÓÉŹĒ_____________________________”£
]£¬øĆĄė×Ó»ÆŗĻĪļ×īæÉÄܵĽį¹¹×é³ÉĪŖ________ (Ģī”°a”±»ņ”°b”±)£¬ĄķÓÉŹĒ_____________________________”£
£Ø5£©¹ĢĢ¬PCl5½į¹¹ÖŠ“ęŌŚPCl![]() ŗĶPCl
ŗĶPCl![]() Į½ÖÖĄė×Ó£¬Ę侧°ūČēĶ¼ĖłŹ¾”£
Į½ÖÖĄė×Ó£¬Ę侧°ūČēĶ¼ĖłŹ¾”£
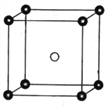
ŅŃÖŖ¾§°ūµÄ±ß³¤ĪŖa nm£¬°¢·ü¼ÓµĀĀŽ³£ŹżÖµÓĆNA±ķŹ¾”£ŌņPCl![]() ŗĶPCl
ŗĶPCl![]() Ö®¼äµÄ×ī¶Ģ¾ąĄėĪŖ_______pm£¬¹ĢĢ¬PCl5µÄĆܶČĪŖ______gcm-3”£
Ö®¼äµÄ×ī¶Ģ¾ąĄėĪŖ_______pm£¬¹ĢĢ¬PCl5µÄĆܶČĪŖ______gcm-3”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŌŚÉśĪļĢåÄŚ£¬Ä³Š©ÖŲŅŖ»ÆŗĻĪļµÄŌŖĖŲ×é³É¼°Ļą»„¹ŲĻµŹ®·ÖĆÜĒŠ”£Ēė¾ŻÓŅĶ¼
·ÖĪöĶź³ÉĻĀĮŠĪŹĢā”£

£Ø1£©Ķ¼ÖŠX”¢Y·Ö±š“ś±ķŗĪÖÖŌŖĖŲ£æX””””””””””””£¬Y”””””””””””””£
£Ø2£©ĒėŌŚBĻĀĆęµÄĄØŗÅÄŚĢīŠ“øĆĄąĪļÖŹµÄĮķĶāČżÖÖ¹¦ÄÜ”£
£Ø3£©BĄąĪļÖŹÓŠ¶ąÖÖ²»Ķ¬¹¦ÄÜ£¬¾æĘäŌŅņŹĒŅņĪŖAĄąĪļÖŹÄŚµÄ””””””””²»Ķ¬”£
£Ø4£©Ļø°ūÄŚŅÅ“«ŠÅĻ¢µÄŠÆ“ųÕߏĒĶ¼ÖŠµÄ””””””””””ĪļÖŹ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠÅÅĮŠĖ³Šņ²»ÕżČ·µÄŹĒ£Ø £©
A.ŌŖĖŲµÄ·Ē½šŹōŠŌ£ŗN£¼O£¼F
B.ĖįŠŌ£ŗHClO4£¾H2SO4£¾H3PO4
C.ČČĪČ¶ØŠŌ£ŗHF£¾HCl£¾H2S
D.Ō×Ó°ė¾¶£ŗAl£¾Mg£¾Na
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ¹ŲÓŚŌ×ÓŠņŹżĪŖ87µÄŌŖĖŲµÄĖµ·ØÕżČ·µÄŹĒ£Ø £©
A.ĖüĪ»ÓŚµŚĮłÖÜĘŚµŚ¢ņA×åB.ĖüĪ»ÓŚµŚĘßÖÜĘŚµŚ¢ņA×å
C.ĖüĪ»ÓŚµŚĘßÖÜĘŚµŚ¢ńA×åD.ĖüµÄŌ×Ó×īĶā²ćÓŠ3øöµē×Ó
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com