
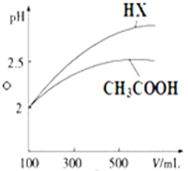
 ��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��
��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | Ka=1.8��10-5 | Kal=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
���� ��1����ͬ�����£���ĵ���ƽ�ⳣ��ԽС������ĵ���̶�ԽС���������ˮ��̶�Խ������ͬŨ�ȵ����Σ���pHֵԽ��
��2����ˮϡ�ʹٽ�������룬��Һ��������Ũ�ȡ��������Ũ�ȡ����������Ũ�ȶ���С���¶Ȳ���ˮ�����ӻ��������䣬����������Ũ������
��3��pH��ȵ����У���ˮϡ�ʹٽ�������룬ϡ����ͬ�ı�����pH�仯���Ϊǿ�ᣬС��Ϊ���
��4��pH=6����Һ��������Ũ��Ϊ10-6mol•L-1�����ݻ��Һ�еĵ���غ���㣮
��� �⣺��1����ͬ�����£���ĵ���ƽ�ⳣ��ԽС������ĵ���̶�ԽС���������ˮ��̶�Խ������ͬŨ�ȵ����Σ���pHֵԽ���ݵ���ƽ�ⳣ��֪���������ˮ��̶ȴ�С˳���ǣ�CO32-��ClO-��HCO3-��CH3COO-�������⼸���ε�pH��С˳���ǣ�a��c��b��d��
�ʴ�Ϊ��a��c��b��d��
��2��A����ˮϡ�ʹٽ�������룬����Һ��c��H+����С����A����
B����ˮϡ�ʹٽ�������룬���������Ӹ�����������Ӹ�����С����$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$����B��ȷ��
C����ˮϡ�ʹٽ�������룬����Һ��������Ũ�ȼ�С���¶Ȳ��䣬ˮ�����ӻ��������䣬����������Ũ����������$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$����C��ȷ��
D���¶Ȳ��䣬����ƽ�ⳣ�����䣬��c��H+��•$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$���䣬��D����
�ʴ�Ϊ��BC��
��3��pH��ȵ����У���ˮϡ�ʹٽ�������룬ϡ����ͬ�ı�����pH�仯���Ϊǿ�ᣬС��Ϊ���ᣬ����HX�����Դ��ڴ��ᣬ��HX�ĵ���ƽ�ⳣ�����ڴ��ᣬ
�ʴ�Ϊ��С�ڣ�
��4��pH=6����Һ�У�������Ũ��Ϊ10-6mol•L-1������������Ũ��Ϊ10-8mol•L-1��������Һ�ʴ��ڵ���غ�c��CH3COO-��+c��OH-��=c��H+��+c��Na+���ɵã�c��CH3COO-��-c��Na+��=c��H+��-c��OH-��=10-6mol•L-1-10-8mol•L-1=9.9��10-7mol•L-1��
�ʴ�Ϊ��9.9��10-7��
���� ���⿼����������ʵĵ��룬��Ŀ�Ѷ��еȣ���ȷ������ʵĵ����ص㡢����ƽ�ⳣ�����������ˮ��̶ȵĹ�ϵ�ٽ���غ�˼������������������ѧ�������Ӧ��������


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ�ƾ���ȡ��ˮ�еĵ� | |
| B�� | Na2O2��ĩ���˵�FeSO4��Һ�У�������ɫ���������ų��������� | |
| C�� | ��Ũ��ˮ�μӵ���ʯ���п��Ƶð�����Ҳ���ü�ʯ�Ҹ��ﰱ�� | |
| D�� | ������KMnO4��Һ��ͨ��SO2��֤SO2��Ư���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
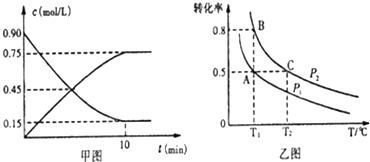
| A�� | ��C��ʾ�÷�Ӧ������Ϊ0.03mol/��L•s�� | |
| B�� | �ﵽƽ�⣬��÷ų�����Ϊx kJ����x=Q | |
| C�� | �����������ٳ���1 mol C�����´ﵽƽ�⣬A������������ֲ��� | |
| D�� | �������¶ȣ���V���棩����V��������С��ƽ�������ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���仯�����й㷺Ӧ�ã�
���仯�����й㷺Ӧ�ã�| NH3•H2O | H2SO3 | ||
| ����ƽ�ⳣ��Ϊ ��mol•L-1�� | 1.7��10-5 | Ka1 | Ka2 |
| 1.54��10-2 | 1.02��10-7 | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com