
��(8��)ijǦ���ص�����������DZ�ĥ��������ͼװ�����ʵ�飬ʶ�����Ǧ���ص���������

(1)��A��E��B��F����B�缫���� ����ӦʽΪ ����˵��FΪ������
(2)ijѧ����ͼ������װ�ã����ݵ缫�����������ʵ��������ⶨ�����ӵ³�����ֵ��ͨ��ʱ��t s���ⶨͨ����·�ĵ���ǿ��ΪI����ȷ���A�缫����ͭ�������IJ����������¼�����
�ٵ��º�ɵ缫�����
��������ˮ��ϴ����ĵ缫
�۳������ǰ�ĵ缫����
���ٴε��º�ɺ����
��ȷ�IJ���˳���� ��
(3)���������������ⶨ����ͭ������Ϊmg�����г����㰢���ӵ������ı���ʽ(��֪һ�����ӵ���Ϊ )��
)��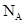 =
=


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��(8��)ijǦ���ص�����������DZ�ĥ��������ͼװ�����ʵ�飬ʶ�����Ǧ���ص���������
(1)��A��E��B��F����B�缫���� ����ӦʽΪ ����˵��FΪ������
(2)ijѧ����ͼ������װ�ã����ݵ缫�����������ʵ��������ⶨ�����ӵ³�����ֵ��ͨ��ʱ��t s���ⶨͨ����·�ĵ���ǿ��ΪI����ȷ���A�缫����ͭ�������IJ����������¼�����
�ٵ��º�ɵ缫�����
��������ˮ��ϴ����ĵ缫
�۳������ǰ�ĵ缫����
���ٴε��º�ɺ����
��ȷ�IJ���˳���� ��
(3)���������������ⶨ����ͭ������Ϊmg�����г����㰢���ӵ������ı���ʽ(��֪һ�����ӵ���Ϊ![]() )��
)��![]() =
= ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�꼪��ʡʵ����ѧ�߶���ѧ����ĩ������⻯ѧ�Ծ� ���ͣ������
(8��)ijͬѧ��Ǧ��������Դ����ʯī���缫���500 mLijŨ�ȵ�CuSO4��Һ���۲쵽A�缫�����к�ɫ�����������ɣ�����Һ��ԭ��������ȫ����ֹͣͨ�磬ȡ��A�缫��ϴ�ӡ�����������缫����1.6 g����֪Ǧ���صĹ���ԭ��Ϊ��
Pb��PbO2��2H2SO4 2PbSO4��2H2O
2PbSO4��2H2O
�밴Ҫ��ش��������⣺
(1)���CuSO4��Һ�Ļ�ѧ����ʽ ��������
(2)����Ӧ������ת�Ƶĵ���Ϊ0.02molʱ��Ǧ������������������ʵ���Ϊ mol��
(3)���ǰCuSO4��Һ�����ʵ���Ũ��Ϊ ��
(4)�����ǰ����Һ��������䣬������Һ��pH=
(5)д��Ǧ���ظ����ĵ缫��Ӧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʦ���и߶���ѧѡ��4��ҵ������������ ���ͣ�ʵ����
��(8��)ijǦ���ص�����������DZ�ĥ��������ͼװ�����ʵ�飬ʶ�����Ǧ���ص���������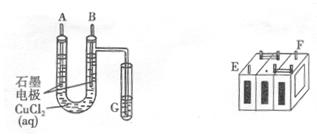
(1)��A��E��B��F����B�缫���� ����ӦʽΪ ����˵��FΪ������
(2)ijѧ����ͼ������װ�ã����ݵ缫�����������ʵ��������ⶨ�����ӵ³�����ֵ��ͨ��ʱ��t s���ⶨͨ����·�ĵ���ǿ��ΪI����ȷ���A�缫����ͭ�������IJ����������¼�����
�ٵ��º�ɵ缫�����
��������ˮ��ϴ����ĵ缫
�۳������ǰ�ĵ缫����
���ٴε��º�ɺ����
��ȷ�IJ���˳���� ��
(3)���������������ⶨ����ͭ������Ϊmg�����г����㰢���ӵ������ı���ʽ(��֪һ�����ӵ���Ϊ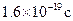 )��
)�� =
= 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ�����и���ģ�⿼�������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
(17��)I����ҵ����һ����CO2�������״�ȼ�ϵķ�����
��6 mol CO2��8 mol H2����2 L���ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ(ʵ��)��ͼ������a(1��6)��ʾ����1 minʱH2�����ʵ�����6 mol��

��1��a������Ӧ���� (����ڡ������ڡ���С�ڡ�)�淴Ӧ���ʡ��仯ѧƽ�ⳣ��K=
(2)����ʱ���ƽ����Ӧ���������� ��
A��O��1 min B��1��3 min C��3��8 min D��8��11 min
(3)���ı�ijһʵ�������ٽ�������ʵ����H2�����ʵ�����ʱ��仯��ͼ��������ʾ������I��Ӧ��ʵ�������ı��� �����ߢ��Ӧ��ʵ�������ı��� ����������ٳ���3molCO2��4 mol H2,H2O(g)��������� ����������䡱��С����
��ijʵ��С����̽�� ��Ӧ�������¶ȵĹ�ϵ,����1mol��L��KI��Һ��0��1mol��L��H2S04��Һ��������Һ����ʵ��ʱ�⼸���Լ��ļ���˳��Ϊ��KI��Һ�� �� ��
��Ӧ�ķ���ʽΪ
��. ������Ʒ�к�Fe��Zn��Ag��Cu�����ֽ������ʣ�Ϊ��øߴ��ȵ�����ij��ȤС��ͬѧ����Ǧ����Ϊ��Դ��������ʯīΪ�缫�������������Һ�Դ��������ᴿ��
(1)�������������������ij������У���Ҫ�Ľ�������Ϊ (�ѧʽ)��

(2) ������ͼ��ʾ���Ӷ�Ǧ���ؽ��г�硣���һ��ʱ�������A�缫������ (�ѧʽ)��B�缫�ϵĵ缫��ӦʽΪ �������ϡ�Ǧ���ص������� ��(�A����B��)��
(3)���ü���ȼ�ϵ��Ϊ��Դ����25�桢101 kPaʱ����CH4��������ֱ��ȼ������1 molˮ��������401 kJ����l gˮ����ת����Һ̬ˮ����2��445 kJ����CH4��ȼ����Ϊ (ȡ����)kJ��mol-��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com