
| A������ | B��ϩ�� | C��Ȳ�� | D������ͬϵ�� |
 CH3OH(g)��H2O(g) ��H����49.0 kJ/mol��
CH3OH(g)��H2O(g) ��H����49.0 kJ/mol��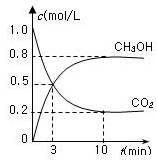
 CO2(g)+ 2H2O(l) ��H1���D 890.3 kJ/mol
CO2(g)+ 2H2O(l) ��H1���D 890.3 kJ/mol H2O(l) ��H2����285.8 kJ/mol
H2O(l) ��H2����285.8 kJ/mol| �ܽ��(S)/g | �ܶȻ�(Ksp) | ||
| Ca(OH)2 | Ba(OH)2 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��2H2(g)+ O 2(g) =" " 2H2O(1) H =" " ��142.9kJ��mol��l H =" " ��142.9kJ��mol��l |
B��2H2(g)+ O 2(g) =" " 2H2O(1) H =" " ��571.6kJ��mol��l H =" " ��571.6kJ��mol��l |
C��2H2+O2 = 2H2O H = ��571.6lkJ��mol��l H = ��571.6lkJ��mol��l |
D��H2(g)+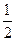 O 2(g) = H2O(1) O 2(g) = H2O(1) H = +285.8kJ��mol��l H = +285.8kJ��mol��l |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 cC(g)+dD(g)����H=Q���ڷ�Ӧ�����У���������������ʱ��ij�����ڻ�����еĺ������¶�(T)��ѹǿ(p)�Ĺ�ϵ����ͼ��ʾ��
cC(g)+dD(g)����H=Q���ڷ�Ӧ�����У���������������ʱ��ij�����ڻ�����еĺ������¶�(T)��ѹǿ(p)�Ĺ�ϵ����ͼ��ʾ��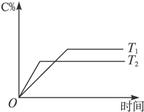
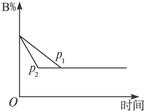
| A��T1>T2��Q>0 | B��T2>T1��Q<0 |
| C��p1>p2��a+b="c+d" | D��p1<p2��b<c+d |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
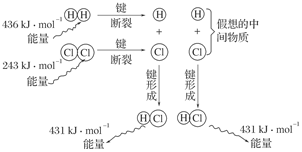
 2HBr(g) ��H=��72kJ/mol������1mol Br2(1)��Ҫ���յ�����Ϊ30kJ����������������±���
2HBr(g) ��H=��72kJ/mol������1mol Br2(1)��Ҫ���յ�����Ϊ30kJ����������������±���| | H2(g) | Br2(g) | HBr(g) |
| ����/kJ��mol��1 | 436 | a | 369 |
 l����ȫȼ�������ȶ�������ų�������ΪY kJ����1molP��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=________________________________��
l����ȫȼ�������ȶ�������ų�������ΪY kJ����1molP��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2.43kJ | B��4.86kJ | C��43.8kJ | D��87.5 kJ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com