
| ���� |
| ���� |
| ���� |
| 1 |
| 5 |
| 5 |
| 8 |
| 5 |
| 8 |
| 3 |
| 8 |
| 2.8��109 |
| 28g/mol |
| 3 |
| 8 |
| 1 |
| 2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��������2007�������ѧ���в��Ի�ѧ�����˽� ���ͣ�038
| |||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ϩ��ʯ�ͻ�������Ҫԭ�ϣ�����Ҫͨ��ʯ�Ͳ�Ʒ�ѽ��á�
��1��ʯ�Ͳ�Ʒ�ѽ������ѽ�����ƽ����ɿɱ�ʾΪCnHm��m>2n�������ⶨij�ѽ����и��������������ֱ�Ϊ������D4%����ϩ�D50%����ϩ�D10%������Ϊ����ϩ�������������������ͬ��ͬѹ�²ⶨ�������õ�50 mol��ϩ��x mol����ϩ��y mol��������x+y= �� n / m = �����ú�x��ʽ�ӱ�ʾ����
��2��ij������ÿ����ʯ�Ͳ�Ʒ�ѽ�õ���ϩ56 t��������ϩΪ��Ҫԭ������������ϩ����������ϩ���Ǻϳ�ά���ڵ���Ҫ���塣����ԭ�����£�
��Ӧ������ϩ��������Ϊ80%����Ӧ������ȩ��������Ϊ83.33%����Ӧ������������ϩ�������ʾ�Ϊ85%����ó�ÿ�������Ƶô�����ϩ�����ٶ֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
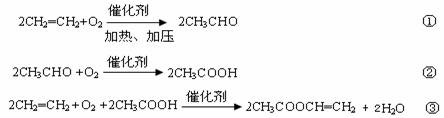
2CH2=CH2+O2![]() 2CH3CHO ��
2CH3CHO ��
2CH3CHO+O2![]() 2CH3COOH ��
2CH3COOH ��
2CH2=CH2+2CH3COOH+O2![]() 2CH3COOCH=CH2+2H2O ��
2CH3COOCH=CH2+2H2O ��
���ڸ���Ӧ�ķ�������Ӧ������ϩ��������Ϊ75%����Ӧ������ȩ��������Ϊ80%����Ӧ�����������ϩ�������ʾ�Ϊ75%��
(1)Ϊʹ������ϩ���������������ϩ�Ⱥ����ε�Ͷ�ϱ�Ϊ���٣�
(2)��2.8��104 kg��ϩΪԭ�������Ƶô�����ϩ������ǧ�ˣ�
(3)�����������Ӧ�з�Ӧ�����ʵ���֮�Ⱦ�������Ӧ��ͬ������ϩ�Ϳ���Ϊԭ������������ϩ����ͨ��Ŀ���(O2���������Ϊ����1/5)����ϩ�����������Ϊ���٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com